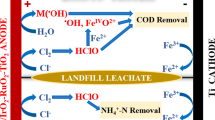
Abstract
The aim of this study is to propose a continuous physicochemical model accounting for the active chlorine production used to degrade recalcitrant sulfamethoxazole (SMX) in an electrochemical flow reactor. The computational model describes the fluid mechanics and mass transfer occurring in the re/actor, along with the electrode kinetics of hydrogen evolution reaction arising on a stainless steel cathode, and the chloride oxidation on a DSA. Specifically, the anodic contributions assume the heterogeneous nature of the adsorbed chlorine species formed on this surface, which are a model requirement to correctly define the experimental reactor performance and degradation efficiency of the contaminant. The experimental validation conducted at different applied current densities, volumetric flows, and chloride concentrations is adequately explained by the model, thus evidencing some of the phenomena controlling the electrocatalytic chlorine production for environmental applications. The best conditions to eliminate the SMX are proposed based on the theoretical analysis of the current efficiency calculated with the model, and experimentally confirmed. The use of the Ti/RuO2-ZrO2-Sb2O3 anode at the bench scale improves the SMX removal by using electro-generated chlorine species adsorbed on its surface, which remarkably increases the oxidation potential of the system along with chlorine desorbed from the electrode. This is a technological innovation concerning other mediated oxidation methods entirely using oxidants in solution.









Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Angulo A, van der Linde P, Gardeniers H, Modestino M, Fernández-Rivas D (2020) Influence of bubbles on the energy conversion efficiency of electrochemical reactors. Joule. 4:555–579. https://doi.org/10.1016/j.joule.2020.01.005
Boreen AL, William A, McNeill K (2004) Photochemical fate of sulfa drugs in the aquatic environment: sulfa drugs containing five-membered heterocyclic groups. Environ Sci Technol. 38:3933-3940. https://doi.org/10.1021/es0353053
Brausch JM, Connors K, Brooks BW, Rand GM (2012) Human pharmaceuticals in the aquatic environment: a review of recent toxicological studies and considerations for toxicity testing. Rev Environ Contam Toxicol 218:1–99. https://doi.org/10.1007/978-1-4614-3137-4
Castañeda LF, Rivera FF, Pérez T, Nava JL (2019) Mathematical modeling and simulation of the reaction environment in electrochemical reactors. Curr Opin Electrochem 16:75–82. https://doi.org/10.1016/j.coelec.2019.04.025
Cheng C, Kelsall G (2007) Models of hypochlorite production in electrochemical reactors with plate and porous anodes. J Appl Electrochem 37(11):1203–1217. https://doi.org/10.1007/s10800-007-9364-7
Cruz-Díaz MR, Rivero EP, Rodríguez FA, Domínguez-Bautista R (2018) Experimental study and mathematical modeling of the electrochemical degradation of dyeing wastewaters in presence of chloride ion with dimensional stable anodes (DSA) of expanded meshes in a FM01-LC reactor. Electrochim Acta 260:726–737. https://doi.org/10.1016/j.electacta.2017.12.025
Dodd MC, Huang CH (2004) Transformation of the antibacterial agent sulfamethoxazole in reactions with chlorine: kinetics, mechanisms, and pathways. Environ Sci Technol 38:5607–5615. https://doi.org/10.1021/es035225z
Elizarragaz D, Carrera-Crespo JE, Vazquez-Arenas J, Romero-Ibarra I, Ruiz-Ruiz EJ, Chairez I (2019) A facile route to synthesize a TiNT-RuO2 electrocatalyst for electro-generated active chlorine production. J Electrochem Soc 166(15):H783–H790. https://doi.org/10.1149/2.0071915jes
Fatta-Kassinos D, Meric S, Nikolaou A (2011) Pharmaceutical residues in environmental waters and wastewater: current state of knowledge and future research. Anal Bioanal Chem 399:251–275. https://doi.org/10.1007/s00216-010-4300-9
Ghoshdastidar AJ, Fox S, Tong AZ (2015) The presence of the top prescribed pharmaceuticals in treated sewage effluents and receiving waters in Southwest Nova Scotia, Canada. Environ Sci Pollut Res 22:689–700. https://doi.org/10.1007/s11356-014-3400-z
Guzmán-Duque FL, Palma-Goyes RE, González I, Peñuela G, Torres-Palma RA (2014) Relationship between anode material, supporting electrolyte and current density during electrochemical degradation of organic compounds in water. J Hazard Mater 278:221–226. https://doi.org/10.1016/j.jhazmat.2014.05.076
Leonzio G (2018) Study of mixing systems and geometric configurations for anaerobic digesters using CFD analysis. Renew Energy 123:578–589. https://doi.org/10.1016/j.renene.2018.02.071
Moreno-Palacios AV, Palma- Goyes RE, Vazquez-Arenas J, Torres-Palma RA (2019) Bench-scale reactor for Cefadroxil oxidation and elimination of its antibiotic activity using electro-generated active chlorine. J Environ Chem Eng 7(3):103173. https://doi.org/10.1016/j.jece.2019.103173
Palma-Goyes RE, Guzmán-Duque FL, Peñuela G, González I, Nava JL, Torres-Palma RA (2010) Electrochemical degradation of crystal violet with BDD electrodes: effect of electrochemical parameters and identification of organic by-products. Chemosphere 81(1):26–32. https://doi.org/10.1016/j.chemosphere.2010.07.020
Palma-Goyes RE, Rivera FF, Vazquez-Arenas J (2019) Heterogeneous model to distinguish the activity of electrogenerated chlorine species from soluble chlorine in an electrochemical reactor. Ind Eng Chem Res 58:22399–22407. https://doi.org/10.1021/acs.iecr.9b05185
Palma-Goyes RE, Silva-Agredo J, Vazquez-Arenas J, Romero-Ibarra I, Torres-Palma RA (2018b) The effect of different operational parameters on the electrooxidation of indigo carmine on Ti/IrO2-SnO2-Sb2O3. J Environ Chem Eng 6(2):3010–3017. https://doi.org/10.1016/j.jece.2018.04.035
Palma-Goyes RE, Vazquez-Arenas J, Ostos C, Ferraro F, Torres-Palma RA, González I (2016b) Microstructural and electrochemical analysis of Sb2O5 doped-Ti/RuO2-ZrO2 to yield active chlorine species for ciprofloxacin degradation. Electrochim Acta 213:740–751. https://doi.org/10.1016/j.electacta.2016.07.150
Palma-Goyes RE, Vazquez-Arenas J, Ostos C, Manzo-Robledo A, Romero-Ibarra I, Calderón JA, González I (2018a) In search of the active chlorine species on Ti/ZrO2-RuO2-Sb2O3 anodes using DEMS and XPS. Electrochim Acta 275:265–274. https://doi.org/10.1016/j.electacta.2018.04.114
Palma-Goyes RE, Vazquez-Arenas J, Ostos C, Torres-Palma RA, González I (2016a) The effects of ZrO2 on the electrocatalysis to yield active chlorine species on Sb2O5-doped Ti/RuO2 anodes. J Electrochem Soc 163:H818–H825. https://doi.org/10.1149/2.0891609jes
Palma-Goyes RE, Vazquez-Arenas J, Torres-Palma RA, Ostos C, Ferraro F, González I (2015) The abatement of indigo carmine using active chlorine electrogenerated on ternary Sb2O5-doped Ti/RuO2-ZrO2 anodes in a filter-press FM01-LC reactor. Electrochim Acta 174:735–744. https://doi.org/10.1016/j.electacta.2015.06.037
Ribeiro J, Moats MS, De Andrade AR (2008) Morphological and electrochemical investigation of RuO2–Ta2O5 oxide films prepared by the Pechini–Adams method. J Appl Electrochem 38(6):767–775. https://doi.org/10.1007/s10800-008-9506-6
Rivera FF, Castañeda L, Hidalgo PE, Orozco G (2017) Study of hydrodynamics at AsahiTM prototype electrochemical flow reactor, using computational fluid dynamics and experimental characterization techniques. Electrochim Acta 245:107–117. https://doi.org/10.1016/j.electacta.2017.05.134
Rivera FF, Ponce de León C, Nava JL, Walsh FC (2015) The filter-press FM01-LC laboratory flow reactor and its applications. Electrochim Acta 163:338–354. https://doi.org/10.1016/j.electacta.2015.02.179
Rivero EP, Rivera FF, Cruz-Díaz MR, Mayen E, González I (2012) Numerical simulation of mass transport in a filter press type electrochemical reactor FM01-LC: Comparison of predicted and experimental mass transfer coefficient. Chem Eng Res Des 90(11):1969–1978. https://doi.org/10.1016/j.cherd.2012.04.010
Verlicchi P, Al Aukidy M, Zambello E (2012) Occurrence of pharmaceutical compounds in urban wastewater: removal, mass load and environmental risk after a secondary treatment-A review. Sci. Total Environ. 429:123-155. https:// doi.org/10.1016/j.scitotenv.2012.04.028
Vogt H, Stephan K (2015) Local microprocesses at gas-evolving electrodes and their influence on mass transfer. Electrochim Acta 155:348–356. https://doi.org/10.1016/j.electacta.2015.01.008
Wang J, Wang S (2018) Microbial degradation of sulfamethoxazole in the environment. Appl Microbiol Biotechnol 102:3573–3582. https://doi.org/10.1007/s00253-018-8845-4
Wang X, Tang D, Zhou J (2007) Microstructure, morphology and electrochemical property of RuO270SnO230 mol% and RuO230SnO270 mol% coatings. J Alloys Compd 430(1-2):60–66. https://doi.org/10.1016/j.jallcom.2006.04.058
Watkinson AJ, Murby EJ, Kolpin DW, Costanzo SD (2009) The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Sci Total Environ 407:2711–2723. https://doi.org/10.1016/j.scitotenv.2008.11.059
Zeng X, Zhang M, Wang X, Chen X, Su X, Tang W (2012) Effects of Sn content on Ti/RuO2–SnO2–TiO2 anodes used in the generation of electrolyzed oxidizing water. J Electroanal Chem 677:133–138. https://doi.org/10.1016/j.jelechem.2012.05.008
Funding
CONACyT “Desarrollo Científico para Atender Problemas Nacionales” grant no. 5604. Financial support was provided by Ciencia Básica CONACYT 2018 grant no. A1-S-21608, SECTEI-CDMX-MEXICO (grant CM 289/2019), SIP-IPN (20200352).
Author information
Authors and Affiliations
Contributions
REP: Draft writing, data curation, performed the experiments and methodology.
FSR: Performed the experiments and methodology, project administration.
FFR: Software, data analysis, conceptualization.
JVA: Data analysis, conceptualization, project administration.
The manuscript was written through the equal contributions of all authors.
All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 829 kb)
Rights and permissions
About this article
Cite this article
Palma-Goyes, .E., Sosa-Rodríguez, F.S., Rivera, F.F. et al. Modeling the sulfamethoxazole degradation by active chlorine in a flow electrochemical reactor. Environ Sci Pollut Res 29, 42201–42214 (2022). https://doi.org/10.1007/s11356-021-16154-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16154-w