Abstract
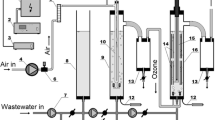
This paper reports the oxidation of aqueous 4-nitrophenol solutions in a photo-electrochemical bubble column reactor (BCR) in which mass transfer has been shown not to be rate limiting. The work represents the first steps in the scale-up of active photoanodes and efficient reactors for the disinfection and detoxification of water.
The preparation, optimization and application of two types of electrode are described and the results are compared with those for a TiO2 electrode supplied by Ineos Chlor. Photocurrents measured in tap water and in aqueous methanol were used for the initial characterization of the electrodes. The methanol was employed for diagnostic purposes only, as discussed below; methanol can react either by direct hole transfer or by hydroxyl radical recombination, but the balance of these reactions depends upon the nature of the electrode surface. The most active thermal electrodes were fabricated by heating titanium metal in air at 750 °C for 10 min, whilst the most active sol–gel electrodes were heated at 600 °C for 10 min.
Three of the central achievements of the work were to: (1) show that it is possible to design and fabricate photoelectrochemical reactors capable of effecting the mineralization of strongly absorbing organics; (2) confirm that the photocatalytic decomposition of 4-NP in reactors with a 4 dm3 capacity can be increased by the application of a small positive potential and (3) that the application of such a potential significantly enhances the mineralization of 4-NP.
For the mineralization of 0.25 mM nitrophenol solutions the reactivity sequence is:
Photoelectrocatalytic > Photocatalytic > Photochemical > Electrochemical.
However, even at 3 V applied potential, charge recombination is not eliminated. The order of electrode activity was:
Ineos > Sol Gel > Thermal.
Differences between the activities of different electrodes were attributed to changes in the structure and morphology of the TiO2. It is noteworthy that although, for nitrophenol oxidation, the thermal electrodes were the least active, for photoelectrocatalytic disinfection in the same type of reactor, thermal electrodes were the most active.
Similar content being viewed by others
References
A. Mills S. Le Hunte (1997) J. Photochem. Photobiol. A 108 1 Occurrence Handle1:CAS:528:DyaK2sXlsFGksLo%3D
P.A. Christensen and G.M. Walker Opportunities for the UK in Solar Detoxification, ETSU s/P4/00249/REP 1996.
D.A. Tryk A. Fujishima K. Honda (2000) Electrochim. Acta 45 2363 Occurrence Handle10.1016/S0013-4686(00)00337-6 Occurrence Handle1:CAS:528:DC%2BD3cXjvFymurw%3D
C.F. Goodeve J.A. Kitchener (1938) Trans. Faraday Soc 34 902 Occurrence Handle1:CAS:528:DyaA1cXmtlyksQ%3D%3D
J.H. Carey J. Lawrence H.M. Tosine (1976) Bull. Environ. Contam. Toxicol 16 697 Occurrence Handle10.1007/BF01685575 Occurrence Handle1:CAS:528:DyaE2sXhs1Kjsrc%3D
A.L. Pruden and D.F. Ollis, J. Catal. 82 (1983) 404; C.Y. Hsia, C.Y. Lee and D.F. Ollis, J. Catal. 82 (1983) 418.
T. Matsunaga R. Tomada T. Nakajima H. Wake (1985) Microbiol. Lett 29 211 Occurrence Handle1:CAS:528:DyaL2MXlsFOhsLg%3D
L. Sun J.R. Bolton (1996) J. Phys. Chem 100 4127 Occurrence Handle1:CAS:528:DyaK28XhtVKktr4%3D
T. Torimoto S. Ito S. Kiwabata H. Yoneyama (1996) Environ. Sci. Technol 30 1275 Occurrence Handle10.1021/es950483k Occurrence Handle1:CAS:528:DyaK28Xht1yks7o%3D
W. Choi J.Y. Ko H. Park J.S. Chung (2001) Appl. Cat. B Environ 31 209 Occurrence Handle1:CAS:528:DC%2BD3MXjtlWjsLw%3D
P. Wyness J.F. Klausner D.Y. Goswami (1994) J. Solar. Eng 116 8 Occurrence Handle1:CAS:528:DyaK2cXks1Clt70%3D
J.C. Crittenden Y. Zhang D.W. Hand (1996) Water Environ. Res 68 270 Occurrence Handle1:CAS:528:DyaK28XjtVOhs7c%3D
L.M. Peter, in R.G. Compton and A. Hamnett (Eds), Comprehensive Chemical Kinetics, Vol. 29 (Elsevier, Amsterdam, 1989), p. 353 and references cited therein.
A. Hamnett, in R.G. Compton (Ed.), Comprehensive Chemical Kinetics, Vol. 27 (Elsevier, Amsterdam, 1987), p. 61.
J. Gautron P. Lemasson J.F. Marucco (1980) Far. Disc. Chem. Soc 70 87
H. Gerischer (1993) Electrochim. Acta 38 3 Occurrence Handle10.1016/0013-4686(93)80003-I Occurrence Handle1:CAS:528:DyaK3sXhtFalu7g%3D
I.M. Butterfield P.A. Christensen A. Hamnett K.E. Shaw G.M. Walker S.A. Walker C.R. Howarth (1997) J. Appl. Electrochem 27 385 Occurrence Handle10.1023/A:1018453402332 Occurrence Handle1:CAS:528:DyaK2sXjtVSjt74%3D
J.C. Harper P.A. Christensen T.A. Egerton T.P. Curtis J. Gunlazuardi (2001) J. Appl. Electrochem 31 623 Occurrence Handle1:CAS:528:DC%2BD3MXlsVemurs%3D
P.A. Christensen T.P. Curtis B. Place G.M. Walker (2002) Water Res 36 2410
K.A. Grey, P. Kamat, U. Stafford and M. Dieckmann, Abstr. Papers Am. Chem. Soc. 203 (1992) 307-ENVR.
B. O’Regan J. Moser M. Anderson M. Gratzel (1990) J. Phys. Chem 94 8720 Occurrence Handle1:CAS:528:DyaK3cXmsVSjs74%3D
B.D. Cullity, Elements of X-ray Diffraction (Addison-Wesley, 1959).
J.C. Harper P.A. Christensen T.A. Egerton K. Scott (2001) Appl. Electrochem 31 267 Occurrence Handle1:CAS:528:DC%2BD3MXivFKgu7Y%3D
T.A. Egerton and P.A. Christensen, in S. Parsons (Ed.), ‘Advanced Oxidation Processes for Water and Wastewater Treatment’ (IWA Publishing, London, 2004), pp. 167–184
D.L. Douglas J. Van Landuyt (1966) Acta Metal 14 491
D. Jiang H. Zhao S. Zhang R. John (2003) J. Phys. Chem 107 12774 Occurrence Handle1:CAS:528:DC%2BD3sXot12ru7k%3D
K. Vinodgopal U. Stafford K.A. Gray P.V. Kamat (1994) J. Phys. Chem 98 6797 Occurrence Handle10.1021/j100078a023 Occurrence Handle1:CAS:528:DyaK2cXksVGjsL8%3D
O.A. Semenikhin V.E. Kazarinov L. Jiang K. Hashimoto A. Fujishima (1999) Langmuir 15 3731 Occurrence Handle10.1021/la981437b Occurrence Handle1:CAS:528:DyaK1MXivVWnsLw%3D
A. Wahl M. Ulmann A. Carroy B. Jermann M. Dolata P. Kedzierzawski C. Chatelain A. Monnier J. Augustynski (1995) J. Electroanal. Chem 396 41 Occurrence Handle10.1016/0022-0728(95)04023-H Occurrence Handle1:CAS:528:DyaK2MXpvV2jsbg%3D
H. Park W. Choi (2004) J. Phys. Chem. B 108 4086 Occurrence Handle1:CAS:528:DC%2BD2cXhvVymsbs%3D
H.Z. Zhang J.F. Banfield (1998) J. Mater. Chem 8 2073 Occurrence Handle1:CAS:528:DyaK1cXlsFKqu7o%3D
E.A. Salinaro N. Serpone (2000) J. Phys. Chem 104 11202
T.A. Egerton C.J. King (1979) J. Oil Col. Chem. Assoc 26 386
E. Pelizzetti V. Maurino C. Minero V. Carlin E. Pramauro O. Zerbinati M.L. Tosato (1990) Environ. Sci. Technol 24 1559 Occurrence Handle10.1021/es00080a016 Occurrence Handle1:CAS:528:DyaK3cXltlyht7s%3D
J.R. Tinlin, Ph. D. Thesis, Newcastle upon Tyne UK (2003).
P.A. Christensen T.P. Curtis T.A. Egerton S.A.M. Kosa J.R. Tinlin (2003) Appl. Cat. B Environ 41 371 Occurrence Handle1:CAS:528:DC%2BD3sXitV2nsrs%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Christensen, P.A., Egerton, T.A., Kosa, S.A.M. et al. The photoelectrocatalytic oxidation of aqueous nitrophenol using a novel reactor. J Appl Electrochem 35, 683–692 (2005). https://doi.org/10.1007/s10800-005-1366-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10800-005-1366-8