Abstract
Aim of study: To investigate if an association exists between in-vivo mucosal levels of IL-8 and bacterial expression of cytotoxin and cagA gene of H. pylori.
Methods: Seventy-two dyspeptic patients referred for endoscopy were studied, including 36 patients with peptic ulcer (PU) and 36 with non-ulcer dyspepsia (NUD). Biopsies were taken for histology, H. pylori culture and measurement of IL-8 by ELISA. To test the ability of H. pylori to produce cytotoxin (VacA), broth culture supernatants were assayed on Vero cells and vacuolation measured. PCR was used to detect the cagA gene of H. pylori.
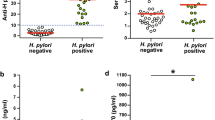
Results: H. pylori was isolated in 52 of 72 patients studied. Among the 52 strains, 25 (49%) were VacA+ve/cagA+ve; 12 (23%) were VacA−ve/cagA−ve; the remaining 15 strains (28%) were either VacA+ve/cagA−ve or VacA−ve/cagA+ve. IL-8 levels (median (interquartile) pg/mg) in patients infected with VacA+ve (1.5 (0.64, 2.84)) or cagA+ve strains (1.25 (0.72, 2.34) were significantly higher than in those with VacA−ve (0.76 (0.4, 1.0)) or cagA−ve strains (0.5 (0.4, 1.5); p<0.05). The neutrophil infiltration score was also higher in patients infected with VacA+ve or cagA+ve strains than in those infected with VacA−ve or cagA−ve strains (p<0.05).
Conclusion: VacA+ve/cagA+ve strains were associated with an enhanced production of mucosal IL-8 in vivo and correlated with a stronger infiltration of neutrophils. Enhanced mucosal production of IL-8 and its role in neutrophil chemotaxis and activation could be important in H. pylori-induced gastroduodenal inflammation and in the development of peptic ulcer disease.
Similar content being viewed by others
References
Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–15.
Rauws EAJ, Tytgat GNT. Eradication of Helicobacter pylori cures duodenal ulcer. Lancet. 1990;335:1233–5.
Morris A, Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol. 1987;82:192–9.
Moss S, Calam J. Helicobacter pylori and peptic ulcers: the present position. Gut. 1992;33:289–92.
Blaser MJ. Hypothesis on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102:720–7.
Blaser MJ, Parsonnet J. Parasitism by the ‘slow’ bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Invest. 1994;94:4–8.
Newell D. The pathogenic mechanism of Helicobacter pylori — a comment. In: Malfertheiner P, Ditschuneit H, eds. Helicobacter pylori, Gastritis and Peptic Ulcer. Berlin: Springer; 1990:128–33.
Figura N, Gutlielmetti P, Rossolinia A et al. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989;27:225–6.
Leunk RD, Johnson PT, David BC, Kraft WG, Morgan DR. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988;26:93–9.
Goossens H, Glupczynski Y, Burette A et al. Role of the vacuolating toxin from Helicobacter pylori in the pathogenesis of duodenal and gastric ulcer. Med Microbiol Lett. 1992;1:153–9.
Atherton JC, Peek RM Jr, Tham KT, Cover TL, Blaser MJ. Clinical and pathological importance of heterogeneity in VacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112:92–9.
Crabtree JE, Taylor JD, Wyatt JL et al. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991;338:332–5.
Cover TL, Blaser MJ. Helicobacter pylori and gastroduodenal disease. Annu Rev Med. 1992;43:135–45.
Davies GR, Banatvala N, Collins CE et al. Relationship between infective load of Helicobacter pylori and reactive oxygen metabolite production in antral mucosa. Scand J Gastroenterol. 1994;29:419–24.
Graham DY. Pathogenic mechanisms leading to Helicobacter pylori induced inflammation. Eur J Gastroenterol Hepatol. 1991;4S:9–16.
Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–9.
Crabtree JE, Lindley IJD. Mucosal interleukin-8 and Helicobacter pylori-associated gastroduodenal disease. Eur J Gastroenterol Hepatol. 1994;6(Suppl. 1):S33–8.
Fan XG, Chua A, Fan XJ, Keeling PMN. Increased gastric production of interleukin-8 and tumour necrosis factor in Helicobacter pylori infection. J Clin Pathol. 1995;48:133–6.
Crabtree JE, Peichl P, Wyatt JI, Stachl U, Lindley IJD. Gastric IL-8 and IL-8 IgA autoantibodies in Helicobacter pylori infection. Scand J Immunol. 1993;37:65–70.
Crabtree JE, Farmery SM, Lindley IJD, Figura N, Peichl P, Tompkins DS. CagA/cytotoxic status of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol. 1994;47:945–50.
Crabtree JE, Covacci A, Farmery SM et al. Helicobacter pylori-induced interleukin 8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995;48:41–5.
Crabtree JE, Xiang Z, Lindley IJD et al. Induction of interleukin-8 secretion from gastric epithelial cells by cagA negative isogenic mutant of Helicobacter pylori. J Clin Pathol. 1995;48:967–9.
Sharma SA, Tummuru MKR, Miller GG, Blaser MJ. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681–7.
Huang JZ, O’Toole PW, Doig P, Trust TJ. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect Immun. 1995;63:1732–8.
Zhang QB, Dawodu JB, Husain A, Etolhi G, Gemmell CG, Russell RI. Association of antral mucosal levels of interleukin 8 and reactive oxygen radicals in patients with Helicobacter pylori. Clin Sci. 1997;92:69–73.
Zhang QB, Nakshabendi IM, Mokhashi MS, Dawodu JB, Gemmell CG, Russell RI. Association of cytotoxin production and neutrophil activation by strains of Helicobacter pylori isolated from patients with peptic ulceration and chronic gastritis. Gut. 1996;38:841–5.
Cover TL, Puryear W, Perez-Perez GI, Blaser MJ. Effect of urease on HaLa cell vacuolation induced by Helicobacter pylori cytotoxin. Infect Immun. 1991;59:1264–70.
Ende AVD, Rauws EAJ, Feller M, Mulder CJJ, Tytgat GNJ, Dankert J. Heterogeneous Helicobacter pylori isolates from members of a family with a history of peptic ulcer disease. Gastroenterology. 1996;111:638–647.
Covacci A, Censini S, Bugnoli M et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5.
Peek RM, Miller GG, Tham KT et al. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest. 1995;73:760–70.
Censini S, Lange C, Xiang Z et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–53.
Xiang Z, Censini S, Bayeli PF et al. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995;63:94–8.
Atherton JC, Cao P, Peek RM Jr, Tummuru MKR, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori: association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–7.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, Q.B., Etolhi, G., Dawodu, J.B. et al. Relationship between mucosal levels of interleukin 8 and toxinogenicity of Helicobacter pylori . Inflammopharmacol 6, 109–117 (1998). https://doi.org/10.1007/s10787-998-0028-y
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10787-998-0028-y