Abstract
Traumatic brain injury (TBI) is an important global health concern that represents a leading cause of death and disability. It occurs due to direct impact or hit on the head caused by factors such as motor vehicles, crushes, and assaults. During the past decade, an abundance of new evidence highlighted the importance of inflammation in the secondary damage response that contributes to neurodegenerative and neurological deficits after TBI. It results in disruption of the blood–brain barrier (BBB) and initiates the release of macrophages, neutrophils, and lymphocytes at the injury site. A growing number of researchers have discovered various signalling pathways associated with the initiation and progression of inflammation. Targeting different signalling pathways (NF-κB, JAK/STAT, MAPKs, PI3K/Akt/mTOR, GSK-3, Nrf2, RhoGTPase, TGF-β1, and NLRP3) helps in the development of novel anti-inflammatory drugs in the management of TBI. Several synthetic and herbal drugs with both anti-inflammatory and neuroprotective potential showed effective results. This review summarizes different signalling pathways, associated pathologies, inflammatory mediators, pharmacological potential, current status, and challenges with anti-inflammatory drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
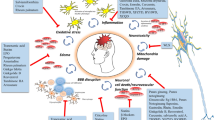
Traumatic brain injury (TBI) is identified as an important global health concern which represents a leading cause of death and disability. TBI occurs due to direct impact or hit on head, caused by a number of things including motor vehicles, crushes, and assaults. The result of initial mechanical damage that happens at the time of injury is referred to as a primary injury. Primary injury is responsible for initiation of secondary injury. Secondary injury develops over a periods of time after primary injury (Hovda et al. 1994; Xiong et al. 1997; Singh et al. 2006). Secondary injury cascades including oxidative stress, endoplasmic reticulum stress, and neuroinflammation contribute to long-term brain damage and can be triggered by a variety of risk factors. These damage cascades converge on an early tau acetylation route, which may act as a catalyst for subsequent degeneration (Lucke-wold et al. 2017). Onset of secondary injury is a result of physiological and biochemical cascades that finally leads to neuronal cell death and functional impairments. Primary injury is an irreversible approach because it is refractory to most therapeutic strategies. It is only prevented by use of safety devices (Werner and Engelhard 2007; Mbye et al. 2008). The interval during which secondary injury develops gives a golden chance for medical approaches that has the ability to prevent and reduce secondary harm while also improving long-term clinical outcomes. However, significant preclinical findings have yet to be validated in clinical trials. Clinical trials have failed due to the physiological variability of trauma patients, as well as a lack of comprehensive pharmacokinetic study for determining the best dosage, starting time of therapy, and therapeutic period of the target drugs (Schouten 2007). As a result, understanding of various molecular and cellular factors which leads to secondary injury is necessary for developing successful neuroprotective approaches for TBI. Neuroinflammation is a secondary damage response that contributes to neurodegenerative and neurological deficits after a TBI. Although most researchers have highlighted negative neuroinflammatory consequences on damaged brain, significant benefits can be obtained if neuroinflammation is treated in a controlled manner. We will look at synthetic and herbal anti-inflammatory drugs that have been explored as therapeutic options for TBI and have shown potential in clinical trials.
Neuroinflammation in pathogenesis of TBI
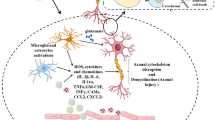
Neuroinflammation plays an important role in neurological impairments and neurodegeneration that might occur after TBI. Increased levels of inflammatory mediators, glial cell activation, and leukocyte recruitment are signs of post-traumatic neuroinflammation (Morganti-Kossmann et al. 2007).
TBI results in disruption of BBB, initiate release of macrophages, neutrophils, and lymphocyte at site of injury. Researchers, working on TBI in animals and humans, have observed an increase in blood-borne immune cells inside the brain parenchyma. Inflammatory mediators are released by these cells, which attract immune and glia cells to the injured area. In addition to immune cell invasion, resident microglia activation plays an important role in damage. Microglial processes form a first-line defence barrier between healthy and injured area of brain (Davalos et al. 2005; Haynes et al. 2006). Whenever microglia become excessively reactive or activated, they release oxidative metabolites (e.g. nitric oxide and reactive oxygen species) as well as pro-inflammatory cytokines [e.g. tumour necrosis factor α (TNF-α), interleukin (IL-1β), and interferon γ (IFN-γ)] that have a detrimental impact on neurons (Block and Hong 2005). Furthermore, production of pro-inflammatory cytokines and supplementary components determines the successive stimulation of astrocytes and glial scar formation in brain injury. Development of intermediary filaments (GFAP and vimentin), elevated cellular accumulation, and cell swelling are all signs of astrocyte activation (Herrmann et al. 2008).
Corresponding to microglia, reactive astrocytes also produce destructive as well as neuroprotective effects in TBI. Activation of astrocyte stimulates variety of neurotrophic factors like brain-derived neurotrophic factor (BDNF) to protect and support brain from cellular death persuaded by injury (Zhao et al. 2004). Moreover, astrocytes are important regulators of extracellular glutamate level and responsible for reducing glutamate excitotoxicity in neurons as well as neuroglia (Schousboe and Waagepetersen 2005). Particularly, damaged astrocytes aggravate transgenic depletion and neuronal deterioration of reactive astrocyte which consequently promotes neuronal death and assists terrible consequences following TBI (Maeda et al. 2003). Hypertrophic astrocytes around lesion site after damage form a suppressive extracellular matrix containing chondroitin sulphate proteoglycans, which prompt the formation of glial scar. The developed strong physiochemical barrier restricts functional connections needed for axonal repair and growth, as well as impedes axonal regeneration (Cafferty et al. 2007). Contrarily, astrocytes give nutritional guidance and support throughout axonal growth after neuronal injury, but chronic astrogliosis limits and impairs functional recovery and axon regeneration (Menet et al. 2003; Wilhelmsson 2004).
Inflammatory signalling pathways in pathogenesis of TBI
Nuclear factor-kappa B (NF-κB) NF-kB is an imperative inflammatory signalling pathway which involves in the synthesis of inflammatory molecules and pro-inflammatory genes such as cytokines and chemokines (Liu et al. 2017b). NF-kB is a downstream element for the stimulation of various receptors such as toll-like receptor 4 (TLR-4) and tumour necrosis factor receptor-associated factor6 (TRAF-6) in human and animals which suffered with TBI. As a consequence, inhibiting NF-kB reduces apoptosis and inflammation following injury. A previous study reported that NF-kB activation in glial and neuronal cells is associated with neuroprotective activity and neurodegenerative diseases (Singh and Singh 2020).In glial cells, NF-kB promotes inflammation, whereas in neurons it plays a role in synaptic plasticity, neuronal development, survival, and synaptic plasticity (Mattson and Camandola 2001). NF-kB levels were found to be higher in rats following fluid percussion and controlled cortical impact head injury, and also in biopsies of human contused neural tissue (Yang et al.1995; McKeating and Andrews 1998).
Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) Pathway JAK/STAT pathway is the fundamental channel intended for transmission of growth factors and cytokines accountable for variety of biochemical processes including axon regeneration, inflammation, cell differentiation, proliferation, and death (Oliva et al. 2012). Activation of JAK-STAT pathway commenced with a particular ligand binding to receptor on cellular surface, which subsequently triggers internal transmission via JAK kinase recruitment. JAK triggers dimerization and expression of STAT components. Some STAT proteins are found in nucleus and regulate gene expression by binding to a specific DNA sequence. After TBI, inflammatory process decreases JAK/STAT expression, resulting in increased cell death in cortical pericontusional region (Oliva et al. 2012). After TBI, rat peri-injured cortex cells were treated with recombinant erythropoietin (rhEPO), which increased JAK2 and STAT3 phosphorylation and reduced apoptosis. The JAK2 inhibitor AG490 lowered pJAK2 and pSTAT3 levels while increasing mRNA expression of many apoptosis-associated genes, implying that JAK2-STAT3 pathway is activated (Zhao et al. 2011) (Table 1).
Mitogen-Activated Protein Kinase (MAPK) Pathway MAPK is a threonine/serine-dependent protein kinase which is triggered by phosphorylation in response to diverse cells trauma. It is important for cell differentiation, proliferation, and survival. Cascades are made up of c-Jun NH (2)-terminal kinase (JNK), extracellular signal-regulated protein kinase (ERK), and p38. Numerous studies reported that activation of p38 and JNK pathway elevates neuronal damage accompanying spinal cord injury and cerebral ischemia (Otani et al. 2002). Following TBI, cell line-based studies revealed astroglial growth and fast ERK activation (Carbonell and Mandell 2003). Pathophysiology of TBI includes abnormalities in the MAPK signalling pathway and studies revealed that blocking this cascade improved cell survival rate and significantly reduces intensity of cortical lesions. JNKs are stress stimulated protein kinases that are found in nucleus of neuronal cells and are linked to neurodegeneration. TBI can activate JNK and cause a complex cascade in mitochondria of brain cells, resulting in apoptosis (Chi et al. 2013; Dietrich and Bramlett 2016) (Fig. 1).
PI3K/Akt/mTOR Signalling Pathway During brain development, the PI3K/Akt/mTOR signalling cascade is an important regulator of neuronal cell proliferation, axon outgrowth, and dendritic formation (Kumar 2005). Various hormones and growth factors that affect mTOR complex and target molecules like mTORC1 and mTORC2 induce downstream Akt and activation of PI3K (Dibble and Cantley 2015). mTOR integrates input from various upstream signals to control cell death, cell growth inhibition, and autophagy. mTOR regulates synthesis of proteins in axons and cell bodies, which are necessary for cell development. TBI-associated symptoms like inflammatory reactions and epilepsy are mostly controlled by inhibiting mTOR pathway (Guo et al. 2013).
Glycogen synthase kinase 3 (GSK-3) Pathway GSK-3 controls protein synthesis, microtubule dynamics, glycogen metabolism, cell differentiation apoptosis, and cell death. Wnt and Akt are two important signalling pathways that regulate GSK-3β activity and are also known as protein kinase B (Fang et al. 2000). Due to Akt activation, phosphorylation of GSK-3β induces response of pro and anti-inflammatory in monocytes. Exclusive GSK-3βinhibitors provide protection to cells from proapoptotic stimulant depending on the role of GSK-3β in apoptosis. Irregular stimulation of GSK-3β is associated with chronic neuroinflammation and neurodegeneration. Several researches have shown that GSK-3β has a role in TBI models of neuroinflammation and also showed the potency of numerous inhibitors of GSK-3βin TBI (Li et al. 2014; Llorens-Marítin et al. 2014).
Nuclear Factor Erythroid 2-Related Factor 2 Pathway Nrf2 is a gene transcription component which protects cells from a variety of damaging stimuli. Nrf2 is mostly found in the cytoplasm, attached to its inhibitor Keap1, which restricts Nrf2 from entering the nucleus. In a recent study, it was found that Nrf2 downregulation promotes neuronal death and neuroinflammation by increasing oxidative stress, TGF-β1, NF-kB, and MMP3/9 (Suzuki and Yamamoto 2017).
Rho-GTPase Pathway Rho-GTPases (Cdc42, Rac1, and RhoA) are principal regulators of cell adhesion and cytoskeletal and cell adhesion controlling a wide order of cellular processes (Chi et al. 2013). Rho GTPase signalling dysregulation has been associated with aetiology of amyotrophic lateral sclerosis (ALS), Alzheimer’s disease, and Parkinson’s disease (Mulherkar and Tolias 2020). Continuous over expression of functional RhoA degrades neuritis repair and axonal regeneration following TBI, since it inhibits axonal regeneration as well as apoptotic responses. Astrocyte activation and proliferation are common responses to CNS damage (Mulherkar and Tolias 2020). The progressive formation of a scar-like structure by astrocytes, oligodendrocytes, microglia fibroblasts, and meningeal cells limits axonal regeneration and slows TBI recovery. In lateral fluid percussion injury model, RhoA activation has been observed in ipsilateral brain of rat (Dubreuil et al. 2006). Furthermore, it was also observed that RhoA activity was increased in neuroglia and spinal cord of rats and mice in rodent models of spinal cord injury models (Wu and Xu 2016).
TGF-β1 Signalling Pathway Microglial inactivation is characterized by TGF-β signalling. TGF-β1 has been shown to play a protective role in CNS diseases in previous research. Taylor et al. discovered that this pathway enhanced functional improvement after intracerebral haemorrhage by altering microglial cell alternative stimulation (Taylor et al. 2016).
Nucleotide-binding domain (NOD)-like receptor protein 3 (NLRP3) Pathway It is a multi-protein complex which aggregates and activates caspase-1 in response to hazardous stimuli and promotes release of the pro-inflammatory cytokines IL-1β and IL-18. Following TBI, immune system activated by these cytokines produces neuroinflammation that contributes to cell death (Lamkanfi et al. 2011).
Anti-inflammatory drugs and TBI
Glucocorticoids and nonsteroidal anti-inflammatory drugs (NSAIDs) are primarily commercially available synthetic anti-inflammatory drugs. Glucocorticoids have potent immunosuppressive and anti-inflammatory properties. They suppress preliminary signs of inflammation like redness, discomfort, and swelling as well as the later stages of wound healing and its proliferative processes that occur in chronic inflammation. Indeed, steroids bind to certain intracellular receptors, forming a complex that later on modulates gene expression, inducing some proteins to be synthesized while others are inhibited (Barnes and Adcock 1993).Glucocorticoids also interact with AP-1, a heterodimer of Fos and Jun proteins that acts as a transcription factor activator. Inhibition of AP-1 inhibits the activity of leukocytes, lymphocytes, and mononuclear cells, as well as the release of pro-inflammatory cytokines including TNF-α and IL-2. Steroids also suppress COX-2 expression, a gene that is ordinarily activated by inflammatory mediators and produces inflammatory prostanoids. In addition, they prevent osteocalcin production in osteoblasts stimulated by vitamin D3. Also, steroids alter collagenase expression and diminish the production of lipocortin 1 (Barnes and Adcock 1993).
NSAID is a class of drugs having combined analgesic, antipyretic, and anti-inflammatory actions due to their inhibitory effect against cyclooxygenase (COX) enzyme. This COX inhibition remarkably reduces the level of IL-1β and also impedes IL-6 synthesis by modulating vasodilator prostaglandin synthesis pathways. Moreover, NSAIDs also inhibit calcium-dependent glutamate release which consequently attenuates glutamate-induced neurotoxicity Breitner 1996).
Most importantly, certain NSAIDs (e.g. aspirin, naproxen, ibuprofen, diclofenac, nabumetone, oxaprozin, and sulindac) also impede COX-1 enzyme causing unwanted adverse effects. However, novel compounds, on the other hand, work selectively on COX-2 and are thought to be more effective. Celecoxib and rofecoxib, two novel anti-inflammatory medicines, are designed to target inflammatory sites alongside protecting non-inflamed areas where COX-mediated prostaglandin generation may be advantageous (Leveugle and Fillit 1994; Lehmann et al. 1997; Ricote et al. 1998).
Another aspect of NSAIDs is that they stimulate the proliferator-activated receptor (PPAR), which causes transcriptional regulatory effects that decrease a variety of pro-inflammatory chemicals and microglial activity. Furthermore, several NSAIDs have antioxidant properties and inhibit NF-kB activation (Grilli et al. 1996) (Table 2).
Anti-inflammatory herbal drugs and TBI
Both crude plant extracts and their isolated compounds have shown neuroprotective effects on nerve functions due to their anti-inflammatory and antioxidant properties. Plant extracts used in traditional system for the alleviation of pain, fever, and inflammation have found to contain several natural anti-inflammatory medicines. A few of these natural products’ processes have been partially explored in recent years, and they are currently considered for therapy of chronic inflammatory and neurodegenerative illnesses. The majority of these medicines work by suppressing COX-2 transcription instead of activity. They also inhibit the expression of a number of pro-inflammatory genes (Keshavarzi et al. 2019).
Recently, numbers of traditional supplements and herbal medicine have been studied in treatment of TBI. Both animal and cellular TBI models revealed elevated expression of NF-κB, TNF-α, IL-6, and IL-1.Treatment with osthol, a coumarin derivative derived from Cnidium monnieri, reduced inflammatory mediators and enhanced neurological functions alongside elevating the neuronal count surrounding the injured area. Furthermore, treatment with osthol lowered the production of numerous inflammatory mediators (Kong et al. 2019).
We evaluated previous study to analyse possible neuroprotective effect of several medicinal plants in brain injury, owing to the increasing number of research published in recent years. Medicinal plants included in Table 3 have shown pharmacological potential in different model of TBI.
Anti-inflammatory drugs and neuroprotective potential in TBI
Anti-inflammatory approaches to prevent and treat neurotoxicity-associated neurological diseases have proved effective in vast cell-based and pre-clinical models, although nothing has been confirmed in later stages of clinical evaluations. However, with conclusive experiments this therapeutic approach propounds encouraging prospects for clinical exploration. In experimental animal models of stroke, both glucocorticoids and general anaesthetic drugs have sparked a great attention in neuroprotection; however, this has yet to be proved in humans (Degos et al. 2022). At some stage of stroke, head trauma, and meningeal bleeding, glucocorticoids have been, however, reported to be ineffective. Classic hypnotics such as thiopental and midazolam have immune-modulating properties and can reduce inflammatory responses in the peripheral nervous system. In an experimental mouse model, they suppress chemotaxis, neutrophil adherence, and phagocytosis as well as impede the discharge of free radicals and pro-inflammatory cytokines; however, these activities have yet to be proven in humans. COX inhibitors (mainly nimesulide and indomethacin) exhibit neuroprotective activity in neonatal mice with brain lesions (Muller 2019). The communication repression between the brain and activated peripheral inflammatory cells through blood–brain barrier induces neuroprotective action. Moreover, COX inhibitors are also reported remarkably effective against depression and various other psychiatric disorders. Noteworthy, celecoxib has been affirmed as an effective drug to treat serious depression and schizophrenia, predominantly in the early stages. Furthermore, acetyl salicylic acid has demonstrated preventative and curative effect against schizophrenia (Degos et al. 2022; Muller 2019).
Current status of anti-inflammatory drugs in management of TBI
Despite advancements in preventative, diagnostic, and surgical techniques, therapeutic choices have been limited for management of TBI. Till date, no pharmacological remedy has been found to provide neuroprotective effects by targeting secondary damage mechanisms (Lozano et al. 2015).
Rehabilitation therapy is used in the majority of the patients. Because damage caused by initial injury is nearly impossible to treat, the rational approach for therapy intervention that provides clinically relevant advantages is to prevent subsequent cell death. It provides a large treatment window due to a delay in harm caused by secondary cell death (Lozano et al. 2015). Targeting neuroinflammation among the secondary wave of biochemical pathways appealed to this extended time for intervention beginning. A variety of medications have been examined and reported to decrease inflammation in animals and TBI patients at the preclinical and clinical levels.
A systematic analysis was performed through US National Institutes of Health clinical trials database using various search strategy consisting of either single or combination of the following keywords: traumatic brain injury, anti-inflammatory drugs, COX-1, COX-2, and specific names of distinct anti-inflammatory drug such as aspirin, celecoxib, ibuprofen, diclofenac. However, no specific findings are obtained in relevance to intervention of anti-inflammatory drugs in prognosis of traumatic brain injury. Although the use of dexamethasone in prognosis of TBI patients with brain contusions and pericontusional oedema is under recruiting status of phase 3, hopefully it will give expected outcomes.
Some clinical evidences suggested certain clinically approved drugs having potential anti-inflammatory activity are used in the treatment of TBI and some are under clinical trials as mentioned in Table 4. Meta-analysis study by Begemann et al. (2020) affirms that TBI patients receiving progesterone, erythropoietin, or cyclosporine have higher chance of a favourable outcome comparatively to those receiving placebo.
Challenges with anti-inflammatory drugs
Clinical trials including anti-inflammatory drugs have produced mixed outcomes so far. Nonselective COX inhibition, inadequate use of specific anti-inflammatory medicines for a given illness or illness progression/severity, sub-optimal dosing at specified location, or inadequate transmission through BBB to brain could all be reasons for varied outcomes (Gilgun-Sherki et al. 2006).
Long-term use of high-dose NSAIDs has been linked to the development of autoimmune disorders. The use of NSAIDs in TBI models is currently investigated; however, the results have been equivocal thus far. In a pre-clinical study, chronic ibuprofen treatment significantly increased cognitive and histological outcome. However, it failed to provide neuroprotection in a TBI model (Harrison et al. 2014).
Prolonged administration of ibuprofen in injured animals provides a much lower results as compared to placebo. Moreover, no significant differences in tissue atrophy level in hippocampus or cortex between treated and untreated mice were observed. These data imply that using high dosages of anti-inflammatory drugs for an extended length of time after trauma may diminish the neuroprotective effects of post-traumatic inflammatory cytokines (Harrison et al. 2014).
Summary
Multiple studies in animal models of TBI have shown that neuroprotective therapies can reduce subsequent damage processes and/or enhance behavioural outcomes. However, none of these promising experimental neuroprotective treatments have been translated to improve clinical outcomes in human. As a result, the development of new anti-inflammatory medications for the treatment of neurodegenerative illnesses that are based on improved BBB transit and have a higher safety profile could result in beneficial therapy. Moreover, the heterogeneity of population genetics and the degree of pathology should be considered while developing novel anti-inflammatory medications. To truly understand the process and chemistry of anti-inflammatory drug delivery into the brain, more research is required. Hence, to decrease this native risk, more specific methods are required to modulate the inflammation. Inflammasome inhibition will be one approach. This technique, however, remains difficult due to a lack of or insufficient understanding of inflammasome structure and activation. Nonetheless, some research on psychiatric disorders has focused at inhibiting NLRP3, the best studied inflammasome. This antagonist endogenously formed after brain injury, and it reduces lesions size in animal models when administered systemically or intracerebrally. To conclude, neuroprotection strategies based on inflammation modulation must maintain the immunological defense and curative functions of inflammation along with eliminating its neurotoxic effects; as a result, three major anti-inflammatory approaches for the neuroprotection pathway can be evolved: modification of peripheral inflammation-CNS communication, modification of pro-inflammatory cytokines–intracerebral targets interaction, and modification of inflammasome production in brain cells.
Availability of data and materials
Information/data collected from open sources.
Abbreviations
- BBB:
-
Blood brain barrier
- BDNF:
-
Bain-derived neurotrophic factor
- COX:
-
Cyclooxygenase
- ERK:
-
Extracellular signal-regulated protein kinase
- GSK-3:
-
Glycogen synthase kinase 3
- IFN-γ:
-
Interferon gamma
- IL-1β:
-
Interleukin 1beta
- JAK/STAT:
-
Janus kinase/signal transducer and activator of transcription
- JNK:
-
C-Jun NH (2)-terminal kinase
- MAPK:
-
Mitogen-activated protein kinase
- MCP-1:
-
Monocyte chemoattractant protein
- NF-κB:
-
Nuclear factor-kappa B
- NLRP3:
-
Nucleotide-binding domain (NOD)-like receptor protein 3
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- PI3K:
-
Phosphatidylinositol-3-kinase
- PPAR:
-
Peroxisome proliferator-activated receptor
- rhEPO:
-
Recombinant human erythropoietin
- TBI:
-
Traumatic brain injury
- TGF-β:
-
Transforming growth factor-beta
- TGF-β1:
-
Transforming growth factor-beta1
- TLR-4:
-
Toll-like receptor 4
- TNF-α:
-
Tumour necrosis factor α
- TRAF-6:
-
Tumour necrosis factor receptor-associated factor 6
References
(2022) In: Academicjournals.org. https://academicjournals.org/article/article1380714402_Wang.pdf. Accessed 18 Apr 2022
(2022) In: Gpb.sav.sk. http://www.gpb.sav.sk/2002_03_231.pdf. Accessed 18 Apr 2022
Anderson G, Peterson T, Vonder Haar C, Kantor E, Farin F, Bammler T, MacDonald J, Hoane M (2013) Comparison of the effects of erythropoietin and anakinra on functional recovery and gene expression in a traumatic brain injury model. Front Pharmacol. https://doi.org/10.3389/fphar.2013.00129
Baez-Jurado E, Vega G, Aliev G, Tarasov V, Esquinas P, Echeverria V, Barreto G (2017) Blockade of neuroglobin reduces protection of conditioned medium from human mesenchymal stem cells in human astrocyte model (T98G) under a scratch assay. Mol Neurobiol 55:2285–2300
Barnes PJ, Adcock I (1993) Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol Sci 14(12):436–441. https://doi.org/10.1016/0165-6147(93)90184-l
Baratz R, Tweedie D, Rubovitch V, Luo W, Yoon J, Hoffer B, Greig N, Pick C (2011) Tumor necrosis factor-α synthesis inhibitor, 3,6′-dithiothalidomide, reverses behavioral impairments induced by minimal traumatic brain injury in mice. J Neurochem 118:1032–1042
Basu A, Krady J, O’Malley M, Styren S, DeKosky S, Levison S (2002) The type 1 interleukin-1 receptor is essential for the efficient activation of microglia and the induction of multiple proinflammatory mediators in response to brain injury. J Neurosci 22:6071–6082
Begemann M, Leon M, van der Horn H, van der Naalt J, Sommer I (2020) Drugs with anti-inflammatory effects to improve outcome of traumatic brain injury: a meta-analysis. Sci Rep. https://doi.org/10.1038/s41598-020-73227-5
Bell M, Kochanek P, Doughty L, Carcillo J, Adelson P, Clark R, Wisniewski S, Whalen M, De Kosky S (1997) Interleukin-6 and interleukin-10 in cerebrospinal fluid after severe traumatic brain injury in children. J Neurotrauma 14:451–457
Bell M, Kochanek P, Carcillo J, Mi Z, Schiding J, Wisniewski S, Clark R, Dixon C, Marion D, Jackson E (1998) Interstitial adenosine, inosine, and hypoxanthine are increased after experimental traumatic brain injury in the rat. J Neurotrauma 15:163–170
Block M, Hong J (2005) Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76:77–98
Bonneh-Barkay D, Zagadailov P, Zou H, Niyonkuru C, Figley M, Starkey A, Wang G, Bissel S, Wiley C, Wagner A (2010) YKL-40 expression in traumatic brain injury: an initial analysis. J Neurotrauma 27:1215–1223
Breitner JC (1996) Inflammatory processes and antiinflammatory drugs in Alzheimer's disease: a current appraisal. Neurobiol Aging 17(5):789–94. https://doi.org/10.1016/0197-4580(96)00109-1
Cafferty W, Yang S, Duffy P, Li S, Strittmatter S (2007) Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J Neurosci 27:2176–2185
Carbonell W, Mandell J (2003) Transient neuronal but persistent astroglial activation of ERK/MAP kinase after focal brain injury in mice. J Neurotrauma 20:327–336
Cernak I, O’Connor C, Vink R (2002) Inhibition of cyclooxygenase 2 by nimesulide improves cognitive outcome more than motor outcome following diffuse traumatic brain injury in rats. Exp Brain Res 147:193–199
Chamoun R, Suki D, Gopinath S, Goodman J, Robertson C (2010) Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J Neurosurg 113:564–570
Chi X, Wang S, Huang Y, Stamnes M, Chen J (2013) Roles of rho GTPases in intracellular transport and cellular transformation. Int J Mol Sci 14:7089–7108
Dash P, Mach S, Moore A (2000) Regional expression and role of cyclooxygenase-2 following experimental traumatic brain injury. J Neurotrauma 17:69–81
Davalos D, Grutzendler J, Yang G, Kim J, Zuo Y, Jung S, Littman D, Dustin M, Gan W (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8:752–758
Degos V, Chhor V, Gressens P, Mantz J (2022) Neuro-inflammation aiguë et strategies neuroprotectrices. Accessed 18 Apr 2022
Dibble C, Cantley L (2015) Regulation of mTORC1 by PI3K signaling. Trends Cell Biol 25:545–555
Dietrich W, Bramlett H (2016) Therapeutic hypothermia and targeted temperature management in traumatic brain injury: clinical challenges for successful translation. Brain Res 1640:94–103
Dubreuil C, Marklund N, Deschamps K, McIntosh T, McKerracher L (2006) Activation of Rho after traumatic brain injury and seizure in rats. Exp Neurol 198:361–369
Engel S, Schluesener H, Mittelbronn M, Seid K, Adjodah D, Wehner H, Meyermann R (2000) Dynamics of microglial activation after human traumatic brain injury are revealed by delayed expression of macrophage-related proteins MRP8 and MRP14. Acta Neuropathol 100:313–322
Fang X, Yu S, Lu Y, Bast R, Woodgett J, Mills G (2000) Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci 97:11960–11965
Gilgun-Sherki Y, Melamed E, Offen D (2006) Anti-inflammatory drugs in the treatment of neurodegenerative diseases: current state. Curr Pharm Des 12:3509–3519
Gohil K, Patel J, Gajjar A (2010) Pharmacological review on Centella asiatica: a potential herbal cure-all. Indian J Pharm Sci 72:546
Grilli M, Pizzi M, Memo M, Spano P (1996) Neuroprotection by aspirin and sodium salicylate through blockade of NF-kappaB activation. Science 274(5291):1383–1385. https://doi.org/10.1126/science.274.5291.1383
Gugliandolo E, D’Amico R, Cordaro M, Fusco R, Siracusa R, Crupi R, Impellizzeri D, Cuzzocrea S, Di Paola R (2018) Neuroprotective effect of artesunate in experimental model of traumatic brain injury. Front Neurol. https://doi.org/10.3389/fneur.2018.00590
Guo D, Zeng L, Brody D, Wong M (2013) Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. PLoS ONE 8:e64078
Hakan T, Toklu H, Biber N, Ozevren H, Solakoglu S, Demirturk P, Aker F (2010) Effect of COX-2 inhibitor meloxicam against traumatic brain injury-induced biochemical, histopathological changes and blood–brain barrier permeability. Neurol Res 32:629–635
Hammad A, Westacott L, Zaben M (2018) The role of the complement system in traumatic brain injury: a review. J Neuroinflammation. https://doi.org/10.1186/s12974-018-1066-z
Harrison J, Rowe R, O’Hara B, Adelson P, Lifshitz J (2014) Acute over-the-counter pharmacological intervention does not adversely affect behavioral outcome following diffuse traumatic brain injury in the mouse. Exp Brain Res 232:2709–2719
Haynes S, Hollopeter G, Yang G, Kurpius D, Dailey M, Gan W, Julius D (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9:1512–1519
He Y, Qu S, Wang J, He X, Lin W, Zhen H, Zhang X (2012) Neuroprotective effects of osthole pretreatment against traumatic brain injury in rats. Brain Res 1433:127–136
Herrmann J, Imura T, Song B, Qi J, Ao Y, Nguyen T, Korsak R, Takeda K, Akira S, Sofroniew M (2008) STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci 28:7231–7243
Hovda D, Fu K, Badie H, Samii A, Pinanong P, Becker D (1994) Administration of an omega-conopeptide one hour following traumatic brain injury reduces 45calcium accumulation. Brain Edema IX 521–523
Hu W, Chan G, Lou J, Wu Q, Wang H, Duan R, Cheng M, Dong T, Tsim K (2018) The extract of Polygoni Cuspidati Rhizoma et Radix suppresses the vascular endothelial growth factor-induced angiogenesis. Phytomedicine 42:135–143
Hutchinson P, O’Connell M, Rothwell N, Hopkins S, Nortje J, Carpenter K, Timofeev I, Al-Rawi P, Menon D, Pickard J (2007) Inflammation in human brain injury: intracerebral concentrations of IL-1α, IL-1β, and their endogenous inhibitor IL-1ra. J Neurotrauma 24:1545–1557
Jassam Y, Izzy S, Whalen M, McGavern D, El Khoury J (2017) Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron 95:1246–1265
Keshavarzi Z, Shakeri F, Barreto G, Bibak B, Sathyapalan T, Sahebkar A (2019) Medicinal plants in traumatic brain injury: Neuroprotective mechanisms revisited. BioFactors 45:517–535
Kong L, Yao Y, Xia Y, Liang X, Ni Y, Yang J (2019) Osthole alleviates inflammation by down-regulating NF-κB signaling pathway in traumatic brain injury. Immunopharmacol Immunotoxicol 41:349–360
Kossmann T, Hans V, Imhof H, Trentz O, Morganti-Kossmann M (1996) Interleukin-6 released in human cerebrospinal fluid following traumatic brain injury may trigger nerve growth factor production in astrocytes. Brain Res 713:143–152
Kossmann T, Stahel P, Morganti-Kossmann M, Jones J, Barnum S (1997) Elevated levels of the complement components C3 and factor B in ventricular cerebrospinal fluid of patients with traumatic brain injury. J Neuroimmunol 73:63–69
Kumar V (2005) Regulation of dendritic morphogenesis by ras-PI3K-Akt-mTOR and ras-MAPK signaling pathways. J Neurosci 25:11288–11299
Kumar A, Rinwa P, Dhar H (2013) Microglial inhibitory effect of ginseng ameliorates cognitive deficits and neuroinflammation following traumatic head injury in rats. Inflammopharmacology 22:155–167
Kunz T, Marklund N, Hillered L, Oliw EH (2022) Effects of the selective cyclooxygenase-2 inhibitor rofecoxib on cell death following traumatic brain injury in the rat. In: PubMed. https://www.ncbi.nlm.nih.gov/pubmed/16518028. Accessed 18 Apr 2022
Lagraoui M, Sukumar G, Latoche JR, Maynard SK, Dalgard CL, Schaefer BC (2017) Salsalate treatment following traumatic brain injury reduces inflammation and promotes a neuroprotective and neurogenic transcriptional response with concomitant functional recovery. Brain Behav Immun 61:96–109. https://doi.org/10.1016/j.bbi.2016.12.005
Laird M, Shields J, Sukumari-Ramesh S, Kimbler D, Fessler R, Shakir B, Youssef P, Yanasak N, Vender J, Dhandapani K (2013) High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia 62:26–38
Lamkanfi M, Walle L, Kanneganti T (2011) Deregulated inflammasome signaling in disease. Immunol Rev 243:163–173
Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA (1997) Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatorydrugs. J Biol Chem 272(6):3406–3410. https://doi.org/10.1074/jbc.272.6.3406
Leveugle B, Fillit H (1994) Proteoglycans and the acutephase response in Alzheimer's disease brain. Mol Neurobiol 9(1–3):25–32. https://doi.org/10.1007/BF02816102
Li D, Liu Z, Wei-Chen M-Y, Li G (2014) Association of glycogen synthase kinase-3β with Parkinson’s disease (Review). Mol Med Rep 9:2043–2050
Li D, Liu N, Zhao H, Zhang X, Kawano H, Liu L, Zhao L, Li H (2017) Interactions between Sirt1 and MAPKs regulate astrocyte activation induced by brain injury in vitro and in vivo. J Neuroinflammation. https://doi.org/10.1186/s12974-017-0841-6
Liu T, Zhang L, Joo D, Sun S (2017a) NF-κB signaling in inflammation. Signal Transduct Target Ther. https://doi.org/10.1038/sigtrans.2017.23
Liu X, Zhao Z, Ji R, Zhu J, Sui Q, Knight G, Burnstock G, He C, Yuan H, Xiang Z (2017b) Inhibition of P2X7 receptors improves outcomes after traumatic brain injury in rats. Purinergic Signalling 13:529–544
Llorens-MarÃtin M, Jurado J, Hernández F, Ãvila J (2014) GSK-3Î2, a pivotal kinase in Alzheimer disease. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2014.00046
Lozano D, Gonzales-Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y, Borlongan CV (2015) Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatric Dis Treatment 8;11:97–106. https://doi.org/10.2147/NDT.S65815
Lucke-Wold B, Seidel K, Udo R, Omalu B, Ornstein M, Nolan R, Rosen C, Ross J (2017) Role of tau acetylation in Alzheimer’s disease and chronic traumatic encephalopathy: the way forward for successful treatment. Journal of Neurology and Neurosurgery 4(2):140
Maas A, Roozenbeek B, Manley G (2010) Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics 7:115–126
Maeda T, Kawane T, Horiuchi N (2003) Statins augment vascular endothelial growth factor expression in osteoblastic cells via inhibition of protein prenylation. Endocrinology 144:681–692
Maier B, Schwerdtfeger K, Mautes A, Holanda M, Müller M, Steudel W, Marzi I (2001) Differential release of interleukines 6, 8, and 10 in cerebrospinal fluid and plasma after traumatic brain injury. Shock 15:421–426
Maier B, Laurer H, Rose S, Buurman W, Marzi I (2005) Physiological levels of pro- and anti-inflammatory mediators in cerebrospinal fluid and plasma: a normative study. J Neurotrauma 22:822–835
Mattson M, Camandola S (2001) NF-κB in neuronal plasticity and neurodegenerative disorders. J Clin Investig 107:247–254
Mbye L, Singh I, Sullivan P, Springer J, Hall E (2008) Attenuation of acute mitochondrial dysfunction after traumatic brain injury in mice by NIM811, a non-immunosuppressive cyclosporin A analog. Exp Neurol 209:243–253
McKeating E, Andrews P (1998) Cytokines and adhesion molecules in acute brain injury. Br J Anaesth 80:77–84
Menet V, Prieto M, Privat A, Ribotta M (2003) Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc Natl Acad Sci 100:8999–9004
Meng X, Li N, Zhang Y, Fan D, Yang C, Li H, Guo D, Pan S (2018) Beneficial effect of β-elemene alone and in combination with hyperbaric oxygen in traumatic brain injury by inflammatory pathway. Transl Neurosci 9:33–37
Momtazi A, Shahabipour F, Khatibi S, Johnston T, Pirro M, Sahebkar A (2016) Curcumin as a MicroRNA regulator in cancer: a review. Rev Physiol Biochem Pharmacol 1–38
Morganti-Kossman M, Lenzlinger P, Hans V, Stahel P, Csuka E, Ammann E, Stocker R, Trentz O, Kossmann T (1997) Production of cytokines following brain injury: beneficial and deleterious for the damaged tissue. Mol Psychiatry 2:133–136
Morganti-Kossmann M, Hans V, Lenzlinger P, Dubs R, Ludwig E, Trentz O, Kossmann T (1999) TGF-β is elevated in the CSF of patients with severe traumatic brain injuries and parallels blood-brain barrier function. J Neurotrauma 16:617–628
Morganti-Kossmann M, Satgunaseelan L, Bye N, Kossmann T (2007) Modulation of immune response by head injury. Injury 38:1392–1400
Mulherkar S, Tolias K (2020) RhoA-ROCK signaling as a therapeutic target in traumatic brain injury. Cells 9:245
Müller N (2019) COX-2 inhibitors, aspirin, and other potential anti-inflammatory treatments for psychiatric disorders. Front Psych. https://doi.org/10.3389/fpsyt.2019.00375
Niswender C, Conn P (2010) Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50:295–322
Oliva A, Kang Y, Sanchez-Molano J, Furones C, Atkins C (2012) STAT3 signaling after traumatic brain injury. J Neurochem 120:710–720
Otani N, Nawashiro H, Fukui S, Nomura N, Yano A, Miyazawa T, Shima K (2002) Differential activation of mitogen-activated protein kinase pathways after traumatic brain injury in the rat hippocampus. J Cereb Blood Flow Metab 22:327–334
Pinteaux E, Parker L, Rothwell N, Luheshi G (2002) Expression of interleukin-1 receptors and their role in interleukin-1 actions in murine microglial cells. J Neurochem 83:754–763
PMC E (2022) Europe PMC. In: Europepmc.org. https://europepmc.org/article/MED/21038663. Accessed 18 Apr 2022
Qin H, Qin J, Hu J, Huang H, Ma L (2017) Malva sylvestris attenuates cognitive deficits in a repetitive mild traumatic brain injury rat model by reducing neuronal degeneration and astrocytosis in the hippocampus. Med Sci Monit 23:6099–6106
Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK (1998) The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391(6662):79–82. https://doi.org/10.1038/34178
Rothwell N (2003) Interleukin-1 and neuronal injury: mechanisms, modification, and therapeutic potential. Brain Behav Immun 17:152–157
Samini F, Samarghandian S, Borji A, Mohammadi G, Bakaian M (2013) Curcumin pretreatment attenuates brain lesion size and improves neurological function following traumatic brain injury in the rat. Pharmacol Biochem Behav 110:238–244
Sawmiller D, Li S, Shahaduzzaman M, Smith A, Obregon D, Giunta B, Borlongan C, Sanberg P, Tan J (2014) Luteolin reduces Alzheimer’s disease pathologies induced by traumatic brain injury. Int J Mol Sci 15:895–904
Schousboe A, Waagepetersen H (2005) Role of astrocytes in glutamate homeostasis: Implications for excitotoxicity. Neurotox Res 8:221–225
Schouten J (2007) Neuroprotection in traumatic brain injury: a complex struggle against the biology of nature. Curr Opin Crit Care 13:134–142
Semple B, Bye N, Rancan M, Ziebell J, Morganti-Kossmann M (2009) Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/− mice. J Cereb Blood Flow Metab 30:769–782
Singh S, Singh T (2020) Role of nuclear factor kappa B (NF-κB) signalling in neurodegenerative diseases: an mechanistic approach. Curr Neuropharmacol 18:918–935
Singh I, Sullivan P, Deng Y, Mbye L, Hall E (2006) Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab 26:1407–1418
Singh P, Singh S, Kapoor I, Singh G, Isidorov V, Szczepaniak L (2013) Chemical composition and antioxidant activities of essential oil and oleoresins from Curcuma zedoaria rhizomes, part-74. Food Biosci 3:42–48
Siopi E, Llufriu-Dabén G, Cho A, Vidal-Lletjós S, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M (2013) Etazolate, an α-secretase activator, reduces neuroinflammation and offers persistent neuroprotection following traumatic brain injury in mice. Neuropharmacology 67:183–192
Stirling D (2004) Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci 24:2182–2190
Stover J, Schöning B, Beyer T, Woiciechowsky C, Unterberg A (2000) Temporal profile of cerebrospinal fluid glutamate, interleukin-6, and tumor necrosis factor-α in relation to brain edema and contusion following controlled cortical impact injury in rats. Neurosci Lett 288:25–28
Suzuki T, Yamamoto M (2017) Stress-sensing mechanisms and the physiological roles of the Keap1–Nrf2 system during cellular stress. J Biol Chem 292:16817–16824
Swartz K, Liu F, Sewell D, Schochet T, Campbell I, Sandor M, Fabry Z (2001) Interleukin-6 promotes post-traumatic healing in the central nervous system. Brain Res 896:86–95
Taylor R, Chang C, Goods B et al (2016) TGF-β1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J Clin Investig 127:280–292
Tehranian R, Andell-Jonsson S, Beni S, Yatsiv I, Shohami E, Bartfai T, Lundkvist J, Iverfeldt K (2002) Improved recovery and delayed cytokine induction after closed head injury in mice with central overexpression of the secreted isoform of the interleukin-1 receptor antagonist. J Neurotrauma 19:939–951
Thau-Zuchman O, Shohami E, Alexandrovich A, Trembovler V, Leker R (2012) The anti-inflammatory drug carprofen improves long-term outcome and induces gliogenesis after traumatic brain injury. J Neurotrauma 29:375–384
Tuttolomondo A, Pecoraro R, Pinto A (2014) Studies of selective TNF inhibitors in the treatment of brain injury from stroke and trauma: a review of the evidence to date. Drug Design Dev Therapy 2221
Vosough-Ghanbari S, Rahimi R, Kharabaf S et al (2010) Effects of Satureja khuzestanica on serum glucose, lipids and markers of oxidative stress in patients with type 2 diabetes mellitus: a double-blind randomized controlled trial. Evid-Based Complement Altern Med 7:465–470
Wang K, Zhang L, Rao W, Su N, Hui H, Wang L, Peng C, Tu Y, Zhang S, Fei Z (2015) Neuroprotective effects of crocin against traumatic brain injury in mice: Involvement of notch signaling pathway. Neurosci Lett 591:53–58
Werner C, Engelhard K (2007) Pathophysiology of traumatic brain injury. Br J Anaesth 99:4–9
Whalen M, Carlos T, Kochanek P, Wisniewski S, Bell M, Clark R, DeKosky S, Marion D, Adelson D (2000) Interleukin-8 is increased in cerebrospinal fluid of children with severe head injury. Crit Care Med 28:929–934
Wilhelmsson U (2004) Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci 24:5016–5021
Winter C (2004) Raised parenchymal interleukin-6 levels correlate with improved outcome after traumatic brain injury. Brain 127:315–320
Winter C, Iannotti F, Pringle A, Trikkas C, Clough G, Church M (2002) A microdialysis method for the recovery of IL-1β, IL-6 and nerve growth factor from human brain in vivo. J Neurosci Methods 119:45–50
Xiong Y, Gu Q, Peterson P, Muizelaar J, Lee C (1997) Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J Neurotrauma 14:23–34
Xu X, Wu X (2016) RhoA/Rho kinase in spinal cord injury. Neural Regen Res 11:23
Xu L, Fagan S, Waller J, Edwards D, Borlongan C, Zheng J, Hill W, Feuerstein G, Hess D (2004) Low dose intravenous minocycline is neuroprotective after middle cerebral artery occlusion-reperfusion in rats. BMC Neurol. https://doi.org/10.1186/1471-2377-4-7
Yang K, Mu X, Hayes R (1995) Increased cortical nuclear factor-κB (NF-κB) DNA binding activity after traumatic brain injury in rats. Neurosci Lett 197:101–104
Yulug B, Kilic E, Altunay S, Ersavas C, Orhan C, Dalay A, Tuzcu M, Sahin N, Juturu V, Sahin K (2018) Cinnamon polyphenol extract exerts neuroprotective activity in traumatic brain injury in male mice. CNS Neurol Disord Drug Targets 17:439–447
Zhang Z, Zhang Z, Artelt M, Burnet M, Schluesener H (2007) Dexamethasone attenuates early expression of three molecules associated with microglia/macrophages activation following rat traumatic brain injury. Acta Neuropathol 113:675–682
Zhao Z, Alam S, Oppenheim R, Prevette D, Evenson A, Parsadanian A (2004) Overexpression of glial cell line-derived neurotrophic factor in the CNS rescues motoneurons from programmed cell death and promotes their long-term survival following axotomy. Exp Neurol 190:356–372
Zhao J, Li G, Zhang Y, Su X, Hang C (2011) The potential role of JAK2/STAT3 pathway on the anti-apoptotic effect of recombinant human erythropoietin (rhEPO) after experimental traumatic brain injury of rats. Cytokine 56:343–350
Funding
The present review did not receive any funding.
Author information
Authors and Affiliations
Contributions
SK, RM, and GS involved in conceptualization; SK, SB, SM took part in original draft preparation; HAM and MA took part in literature review; SK, RM, GS, SB, AA took part in writing, review, and editing; LA, GS, and SB took part in proof reading.
Corresponding authors
Ethics declarations
Competing interests
The author(s) declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
All the authors have approved the manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kalra, S., Malik, R., Singh, G. et al. Pathogenesis and management of traumatic brain injury (TBI): role of neuroinflammation and anti-inflammatory drugs. Inflammopharmacol 30, 1153–1166 (2022). https://doi.org/10.1007/s10787-022-01017-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-022-01017-8