Abstract
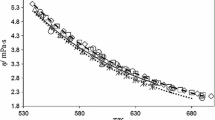
The vibrating-wire viscometer has proven to be an exceedingly effective means of determining the viscosity of liquids over a wide range of temperature and pressure. The instrument has a long history but a variety of technological and theoretical developments over a number of years have improved its precision and most recently have enabled absolute measurements of high accuracy. However, the nature of the electrical measurements required for the technique has inhibited its widespread use for electrically conducting liquids so that there have been only a limited number of measurements. In the particular context of ionic liquids, which have themselves attracted considerable attention, this is unfortunate because it has meant that one primary measurement technique has seldom been employed for studies of their viscosity. In the last 2 years systematic efforts have been made to explore the applicability of the vibrating-wire technique by examining a number of liquids of increasing electrical conductivity. These extensions have been successful. However, in the process we have had cause to review previous studies of the viscosity and density of the same liquids at moderate temperatures and pressures and significant evidence has been accumulated to cause concern about the application of a range of viscometric techniques to these particular fluids. Because the situation is reminiscent of that encountered for a new set of environmentally friendly refrigerants at the end of the last decade, in this paper the experimental methods employed with these liquids have been reviewed which leads to recommendations for the handling of these materials that may have consequences beyond viscometric measurements. In the process new viscosity and density data for 1-hexyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide [\({\mathrm{C}}_{6}\)mim][\({\mathrm{NTf}}_{2}\)], 1-ethyl-3-methylimidazolium ethyl sulfate [\({\mathrm{C}}_{2}\)mim][\({\mathrm{EtSO}}_{4}\)], and 1-ethyl-3-methylpyridinium ethyl sulfate [\({\mathrm{C}}_{2}\)mpy][\({\mathrm{EtSO}}_{4}\)] have been obtained.









Similar content being viewed by others
References
G. Maitland, M. Rigby, E.B. Smith, W.A. Wakeham, Intermolecular Forces: Their Origin and Determination (Clarendon Press, Oxford, 1987)
W.A. Wakeham, A. Nagashima, J.V. Sengers (eds.), Experimental Thermodynamics, Measurement of the Transport Properties of Fluids, vol. III (Blackwell Scientific for International Union of Pure and Applied Chemistry, Oxford, 1991)
Achievements in Stratospheric Ozone Protection: Progress Report (U.S. Environmental Protection Agency, Office of Air and Radiation, April 2007)
Ozone Secretariat, The Montreal Protocol on Substances that Deplete the Ozone Layer, as either adjusted and/or amended in London 1990; Copenhagen 1992; Vienna 1995; Montreal 1997; Beijing 1999, United Nations Environment Program, March 2000
Office for Official Publications of the European Communities, Montreal Protocol: Air Environment (European Commission, 2007)
M.J. Assael, W.A. Wakeham, Int. J. Refrig. 18, 335 (1995)
C.A. Nieto de Castro, J. Mol. Liq. 156, 10 (2010)
K. Marsh, J.F. Brennecke, R.D. Chirico, M. Frenkel, A. Heintz, J.W. Magee, C.J. Peters, L.P.N. Rebelo, K.R. Seddon, Pure Appl. Chem. 81, 781 (2009)
R.D. Chirico, V. Diky, J.W. Magee, M. Frenkel, K. Marsh, Pure Appl. Chem. 81, 791 (2009)
C.A. Nieto de Castro, F.J.V. Santos, J.M.N.A. Fareleira, W.A. Wakeham, J. Chem. Eng. Data 54, 171 (2009)
W.A. Wakeham, M.A. Assael, J.K. Atkinson, J. Bilek, J.M.N.A. Fareleira, A.D. Fitt, A.R.H. Goodwin, C.M.B.P. Oliveira, Int. J. Thermophys. 28, 372 (2007)
J.L. Copeland, Transport Properties of Ionic Liquids (Gordon and Breach, New York, 1974)
B.D. Fitchett, T.N. Knepp, J.C. Conboy, J. Electrochem. Soc. 151, E219 (2004)
J.M. Crosthwaite, M.J. Muldoon, J.K. Dixon, J.L. Anderson, J.F. Brennecke, J. Chem. Thermodyn. 37, 559 (2005)
H. Tokuda, K. Hayamizu, K. Ishii, M.A.B.H. Susan, M. Watanabe, J. Phys. Chem. B 109, 6103 (2005)
H. Tokuda, S. Tsuzuki, M. Susan, K. Hayamizu, M. Watanabe, J. Phys. Chem. B 110, 19593 (2006)
A. Muhammad, M.I.A. Mutalib, C.D. Wilfred, T. Murugesan, A. Shafeeq, J. Chem. Thermodyn. 40, 1433 (2008)
A. Ahosseini, A.M. Scurto, Int. J. Thermophys. 29, 1222 (2008)
J.A. Widegren, J.W. Magee, J. Chem. Eng. Data 52, 2331 (2007)
F.J.V. Santos, C.A. Nieto de Castro, P.J.F. Mota, A.P.C. Ribeiro, Int. J. Thermophys. 31, 1869 (2010)
M.E. Kandil, K.N. Marsh, A.R.H. Goodwin, J. Chem. Eng. Data 52, 2382 (2007)
J.C.F. Diogo, F.J.P. Caetano, J.M.N.A. Fareleira, W.A. Wakeham, Fluid Phase Equilib. 353, 76 (2013). doi:10.1016/j.fluid.2013.05.012
K.N. Marsh, Pure Appl. Chem. 72, 1809 (2000)
M. Kwata, K. Kurase, A. Nagashima, K. Yoshida, in Measurement of the Transport Properties of Fluids, vol. III, ed. by W.A. Wakeham, A. Nagashima, J.V. Sengers (Blackwell Scientific Publications, Oxford, 1991) pp. 7–48
H. Bauer, G. Meerlender, Rheol. Acta 23, 514 (1984)
F.J.P. Caetano, J.M.N.A. Fareleira, A.C. Fernandes, C.M.B.P. Oliveira, A.P. Serro, I.M. Simões de Almeida, W.A. Wakeham, Fluid Phase Equilib. 245, 1 (2006)
Anton Paar, GmbH; Stabinger Viscometer: Pressen Product. http://www.anton-paar.com/001/en/Web/Document/download/1603?clng=en. Accessed 8 Sept 2012
Anton Paar, GmbH; Stabinger Viscometer. http://www.anton-paar.com/001/en/Web/Document/download/1605?clng=en. Accessed 8 Sept 2012
Anton Paar, GmbH; SVM 3000 Stabinger Viscometer. http://www.anton-paar.com/001/en/Web/Document/download/1039?clng=en. Accessed 8 Sept 2012
K.R. Seddon, A. Stark, M.J. Torres, Pure Appl. Chem. 72, 2275 (2000)
C.A. Nieto de Castro, E. Langa, A.L. Morais, M.L.M. Lopes, M.J.V. Lourenco, F.J.V. Santos, M. Santos, J.N.C. Lopes, H.I.M. Veiga, M. Macatrao, J. Esperanca, C.S. Marques, L.P.N. Rebelo, C.A.M. Afonso, Fluid Phase Equilib. 294, 157 (2010)
F.J.P. Caetano, J.M.N.A. Fareleira, C.M.B.P. Oliveira, W.A. Wakeham, J. Chem. Eng. Data 50, 201 (2005)
J.C.F. Diogo, F.J.P. Caetano, J.M.N.A. Fareleira, W.A. Wakeham, C.A.M. Afonso, C.S. Marques, J. Chem. Eng. Data 57, 1015 (2012)
T. Retsina, S.M. Richardson, W.A. Wakeham, Appl. Sci. Res. 43, 325 (1987)
A.A.H. Pádua, J.M.N.A. Fareleira, J.C.G. Calado, W.A. Wakeham, Int. J. Thermophys. 17, 781 (1996)
J. Vaughan, J. Haggins, D. Dreisinger, ECS Trans. 2, 381 (2006)
J. Vila, P. Gines, J.M. Pico, C. Franjo, E. Jimenez, Fluid Phase Equilib. 242, 141 (2006)
Conductivity Standard 0.01D, 1408 \(\mu \)S/cm \(\pm \) 0.5% @ \(25^{\circ }\)C, manufactured by HACH LANGE GmbH for Radiometer Analytical SAS, Serial Number C01622, Calibration Mark 000335/DKD-K-47901/10-03 (Villeurbanne Cedex, France, 2010)
Conductivity Standard 0.1D, 12.85 mS/cm \(\pm \) 0.35% @ \(25^{\circ }\)C, manufactured by HACH LANGE GmbH for Radiometer Analytical SAS, Serial Number C01600, Calibration Mark 000313/DKD-K-47901/10-03 (Villeurbanne Cedex, France, 2010)
R.A. Robinson, R.H. Stokes, Electrolyte Solutions, 2nd edn. (Butterworths, London, 1959)
F.J.P. Caetano, J.M.N.A. Fareleira, A.P. Fröba, K.R. Harris, A. Leipertz, C.M.B.P. Oliveira, J.P. Martin Trusler, W.A. Wakeham, J. Chem. Eng. Data 53, 2003 (2008)
P.J. Carvalho, M.G. Freire, I.M. Marrucho, A.J. Queimada, J.A.P. Coutinho, J. Chem. Eng. Data 53, 1346 (2008)
J. Restolho, J.L. Mata, B. Saramago, J. Colloid Interface Sci. 340, 82 (2009)
Acknowledgments
This work was developed under Projects PTDC/QUI/66826/2006 and PTDC/EQU-EPR/103505/2008 and was also partially supported by the Multiannual Funding to Centro de Química Estrutural and by the Strategic Project PEst-OE/QUI/UI0100/2011, all funded by Fundação para a Ciência e a Tecnologia (FCT, Portugal). The authors acknowledge the permission of Elsevier B.V. to reproduce in this article (see Tables 1, 2) Tables 6a and 6b of the paper “Viscosity Measurements of Three Ionic Liquids Using the Vibrating Wire Technique”, taken from Ref. [22]. The authors also acknowledge the grant attributed to J.C.F.D. under the above mentioned project PTDC/66826/2006. Furthermore, J.C.F.D. thanks FCT, Portugal, for his PhD grant (SFRH / BD / 66736 / 2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diogo, J.C.F., Caetano, F.J.P., Fareleira, J.M.N.A. et al. Viscosity Measurements on Ionic Liquids: A Cautionary Tale. Int J Thermophys 35, 1615–1635 (2014). https://doi.org/10.1007/s10765-013-1487-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-013-1487-y
Keywords
- 1-Ethyl-3-methylpyridinium ethyl sulfate [\({\mathrm{C}}_{2}\)mim][\({\mathrm{EtSO}}_{4}\)]
- 1-Ethyl-3-methylpyridinium ethyl sulfate [\({\mathrm{C}}_{2}\)mpy][\({\mathrm{EtSO}}_{4}\)]
- 1-Hexyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide\([{\mathrm{C}}_{6}{\mathrm{mim}}][{\mathrm{NTf}}_{2}\)]
- Ionic liquids
- Viscosity