Abstract
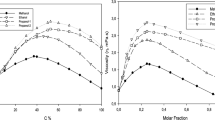
A simple extension to the undergraduate laboratory experiment for the determination of viscosity using Ostwald’s viscometer is proposed in this work. This fits in well for any undergraduate laboratory demonstration. Ostwald’s viscometer is used to investigate the effect of concentration, temperature, and intermolecular interactions on the viscosity of liquids using distilled water as the reference liquid. The viscosity values obtained for acetic acid (v/v%) and sugar solutions (w/v%) have been found to increase with an increase in the concentration of solutions and are synchronous with the literature trends. A drastic decrease in viscosity is observed with an increase in temperature due to a decrease in intermolecular forces of attraction. The temperature variation is found to be a more dominating and contributing factor towards changing viscosity values as compared to the concentration variation. Liquids with stronger intermolecular interactions are found to have greater viscosity values. However, this result was found to be dominated by the factor of greater molecular mass. Nonetheless, the trends obtained in this work are synchronous with literature data.
Similar content being viewed by others
Suggested Reading
L Korson, W Drost-Hansen, and J F Millero, Viscosity of water at various temperatures, J. Phys. Chem., Vol.73, No.1, pp.34–39, 1969.
R Alcalde, G Garcia, M Atilhan and S Aparicio, Systematic study on the viscosity of ionic liquids: Measurement and prediction, Ind. Eng. Chem. Res., Vol.54, No.43, pp.10918–10924, 2015.
G Barr, A Monograph of Viscosity, Oxford University Press, London, 1931.
J R van Wazer, J W Lyons, K Y Kim and R E Colwell, Viscosity and Flow Measurement, Interscience, New York, 1963.
H White and E A Kearsley, An absolute determination of viscosity using a torsional pendulum, J. Res. Nat. Bur. Stand.(U.S.) 75A, (Phys. and Chem.), Vol.6, pp.553–560, 1971.
M Maheshwar, A review article on measurement of viscosity, IJRPC, Vol.8, No.1, pp.69–77, 2018.
J G Webster, The Measurement, Instrumentation and Sensors Handbook, CRC Press LLC, USA, 2000.
R S Marvin, The accuracy of measurements of viscosity of liquids, J. Res. Natl. Bur. Stand. (U. S.) 75A, (Phys. and Chem.), Vol.6, pp.535–540, 1971.
O Prezhdo, A Drogosz, V Zubkova and V Prezhdo, On viscosity of selected normal and associated liquids, J. Mol. Liq., Vol.182, pp.32–38, 2013.
R Simbha, Effect of concentration on the viscosity of dilute solutions, J. Res. Natl. Bur. Stand., (U.S.), Vol.42, pp.409–418, 1949.
Acknowledgements
The authors are grateful to Prof. (Dr.) John Varghese, Principal, St. Stephen’s College; Dr. Shabnam Johry, Department of Chemistry, St. Stephen’s College and Chemistry Laboratory Support, Department of Chemistry, St. Stephen’s College, for their encouragement and endless support.
Funding
This work was supported by St. Stephen’s College, University of Delhi, India.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Thakral, P., Ingle, J.B., Raina, R. et al. Study of Variations of Concentration, Temperature, and Intermolecular Interactions on the Viscosity of Liquids. Reson 27, 1789–1803 (2022). https://doi.org/10.1007/s12045-022-1472-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12045-022-1472-5