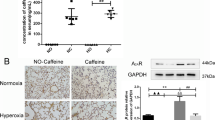
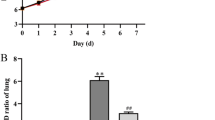
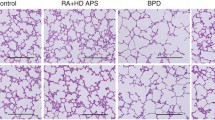
Abstract
Bronchopulmonary dysplasia (BPD) is a common devastating pulmonary complication in preterm infants. Supplemental oxygen is a lifesaving therapeutic measure used for premature infants with pulmonary insufficiency. However, oxygen toxicity is a significant trigger for BPD. Oxidative stress disrupts lung development, accompanied by increased pro-inflammatory cytokines and chemokines expression and immune cells infiltration in lung tissue. Licorice, a typical traditional herbal medicine, is commonly used in the medicine and food industries. 18β-Glycyrrhetinic acid (18β-GA), a primary active ingredient of licorice, has powerful anti-oxidative and anti-inflammatory effects. This study aimed to determine whether 18β-GA has a protective effect on neonatal rats with hyperoxia exposure. Newborn Sprague–Dawley rats were kept in either 21% (normoxia) or 80% O2 (hyperoxia) continuously from postnatal day (PN) 1 to 14. 18β-GA was injected intragastrically at 50 or 100 mg/kg body weight once a day from PN 1 to 14. We examined the body weight and alveolar development and measured ROS level and the markers of pulmonary inflammation. Mature-IL-1β and NF-κB pathway proteins, and the NLRP3 inflammasome, were assessed; concurrently, caspase-1 activity was measured. Our results indicated that hyperoxia resulted in alveolar simplification and decreased bodyweight of neonatal rats. Hyperoxia increased ROS level and pulmonary inflammation and activated NF-κB and the NLRP3 inflammasome. 18β-GA treatment inhibited the activation of NF-κB and the NLRP3 inflammasome, decreased ROS level and pulmonary inflammation, improved alveolar development, and increased the bodyweight of neonatal rats with hyperoxia exposure. Our study demonstrates that 18β-GA has a protective effect on neonatal rats with hyperoxia exposure.









Similar content being viewed by others
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 18β-GA:
-
18β-Glycyrrhetinic acid
- ASC:
-
Apoptosis-associated Specklike protein containing CARD
- BPD:
-
Bronchopulmonary dysplasia
- CINC-1:
-
Cytokine-induced neutrophil chemoattractant-1
- DCFH-DA:
-
Dichlorodihydrofluorescein diacetate
- ELISA:
-
Enzyme-linked immunosorbent assay
- MIF:
-
Macrophage migration inhibitory factor
- MIP-2:
-
Macrophage inflammatory protein-2
- MPO:
-
Myeloperoxidase
- NAC:
-
N-acetyl-L-cysteine
- NAG:
-
N-Acetyl-β-D-glucosaminidase
- NF-κB:
-
Nuclear factor-κB
- NLRP3:
-
NOD-, LRR-, and pyrin-domain containing protein 3
- PN:
-
Postnatal day
- PVDF:
-
Polyvinylidene fluoride
- RAC:
-
Radial alveolar count
- ROS:
-
Reactive oxygen species
- SD:
-
Sprague-Dawley
- TBST:
-
Tris-buffered saline and Tween-20
- Th2:
-
T helper type 2
- TNF:
-
Tumor necrosis factor
References
Abman, S.H., E. Bancalari, and A. Jobe. 2017. The evolution of bronchopulmonary dysplasia after 50 years. American Journal of Respiratory and Critical Care Medicine 195: 421–424. https://doi.org/10.1164/rccm.201611-2386ED.
Lignelli, E., F. Palumbo, D. Myti, and R.E. Morty. 2019. Recent advances in our understanding of the mechanisms of lung alveolarization and bronchopulmonary dysplasia. American Journal of Physiology. Lung Cellular and Molecular Physiology 317: L832–L887. https://doi.org/10.1152/ajplung.00369.2019.
Principi, N., G. Di Pietro, and S. Esposito. 2018. Bronchopulmonary dysplasia: Clinical aspects and preventive and therapeutic strategies. Journal of Translational Medicine 16: 36. https://doi.org/10.1186/s12967-018-1417-7.
Hwang, J.S., and V.K. Rehan. 2018. Recent advances in bronchopulmonary dysplasia: Pathophysiology, prevention, and treatment. Lung 196: 129–138. https://doi.org/10.1007/s00408-018-0084-z.
Kalikkot, T.R., M. Guaman, and B. Shivanna. 2017. Bronchopulmonary dysplasia: A review of pathogenesis and pathophysiology. Respiratory Medicine 132: 170–177. https://doi.org/10.1016/j.rmed.2017.10.014.
Baraldi, E., and M. Filippone. 2007. Chronic lung disease after premature birth. New England Journal of Medicine 357: 1946–1955. https://doi.org/10.1056/NEJMra067279.
Gough, A., M. Linden, D. Spence, C.C. Patterson, H.L. Halliday, and L.P.A. McGarvey. 2014. Impaired lung function and health status in adult survivors of bronchopulmonary dysplasia. European Respiratory Journal 43 (3): 808–816. https://doi.org/10.1183/09031936.00039513.
Jensen, E.A., and B. Schmidt. 2014. Epidemiology of bronchopulmonary dysplasia. Birth Defects Research. Part A, Clinical and Molecular Teratology 100: 145–157. https://doi.org/10.1002/bdra.23235.
Lewandowski, A.J., W.M. Bradlow, D. Augustine, E.F. Davis, J. Francis, A. Singhal, A. Lucas, S. Neubauer, K. McCormick, and P. Leeson. 2013. Right ventricular systolic dysfunction in young adults born preterm. Circulation 128 (7): 713–720. https://doi.org/10.1161/CIRCULATIONAHA.113.002583.
Nakanishi, H., A. Uchiyama, and S. Kusuda. 2016. Impact of pulmonary hypertension on neurodevelopmental outcome in preterm infants with bronchopulmonary dysplasia: A cohort study. Journal of Perinatology 36 (10): 890–896. https://doi.org/10.1038/jp.2016.108.
Sipola-Leppänen, M., M. Vääräsmäki, M. Tikanmäki, P. Hovi, S. Miettola, A. Ruokonen, A. Pouta, M. Järvelin, and E. Kajantie. 2014. Cardiovascular risk factors in adolescents born preterm. Pediatrics 134 (4): e1072–e1081. https://doi.org/10.1542/peds.2013-4186.
Sriram, S., Schreiber, M. D., Msall, M. E., Kuban, K. C., Joseph, R. M., O’Shea, T. M., Allred, E.N., Leviton, A., ELGAN Study Investigators. 2018. Cognitive development and quality of life associated with BPD in 10-year-olds born preterm. Pediatrics, 141(6), undefined. https://doi.org/10.1542/peds.2017-2719.
Wilson-Costello, D., M.C. Walsh, J.C. Langer, R. Guillet, A.R. Laptook, B.J. Stoll, S. Shankaran, N.N. Finer, M.K.P. Van, W.A. Engle, et al. 2009. Impact of postnatal corticosteroid use on neurodevelopment at 18 to 22 months’ adjusted age: Effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics 123 (3): e430–e437. https://doi.org/10.1542/peds.2008-1928.
Doyle, L.W., Cheong, J.L., Ehrenkranz, R.A., Halliday, H.L. 2017. Early (< 8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev, 10(undefined), CD001146. https://doi.org/10.1002/14651858.CD001146.pub5.
Isayama, T., H. Iwami, S. McDonald, and J. Beyene. 2016. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: A systematic review and meta-analysis. JAMA 316 (6): 611–624. https://doi.org/10.1001/jama.2016.10708.
Sweet, D.G., V. Carnielli, G. Greisen, M. Hallman, E. Ozek, A. Te Pas, R. Plavka, C.C. Roehr, D. Saugstad Ola, U. Simeoni, et al. 2019. European consensus guidelines on the management of respiratory distress syndrome - 2019 update. Neonatology 115 (4): 432–450. https://doi.org/10.1159/000499361.
Saugstad Ola, D. 2018. Oxygenation of the immature infant: A commentary and recommendations for oxygen saturation targets and alarm limits. Neonatology 114 (1): 69–75. https://doi.org/10.1159/000486751.
Poggi, C, Dani, C. 2014. Antioxidant strategies and respiratory disease of the preterm newborn: an update. Oxid Med Cell Longev, (undefined), 721043. https://doi.org/10.1155/2014/721043.
Bhandari, V. 2010. Hyperoxia-derived lung damage in preterm infants. Seminars in Fetal and Neonatal Medicine 15 (4): 223–229. https://doi.org/10.1016/j.siny.2010.03.009.
Morty, R.E. 2018. Recent advances in the pathogenesis of BPD. Seminars in Perinatology 42 (7): 404–412. https://doi.org/10.1053/j.semperi.2018.09.001.
Wang, J., Dong, W. 2018. Oxidative stress and bronchopulmonary dysplasia. Gene, 678(undefined), 177–183. https://doi.org/10.1016/j.gene.2018.08.031.
Berger, J., Bhandari, V. 2014. Animal models of bronchopulmonary dysplasia. The term mouse models. Am J Physiol Lung Cell Mol Physiol, 307: L936–47. https://doi.org/10.1152/ajplung.00159.2014.
Buczynski, B.W., E.T. Maduekwe, and M.A. O’Reilly. 2013. The role of hyperoxia in the pathogenesis of experimental BPD. Seminars in Perinatology 37 (2): 69–78. https://doi.org/10.1053/j.semperi.2013.01.002.
Nardiello, C., I. Mižíková, and R.E. Morty. 2017. Looking ahead: Where to next for animal models of bronchopulmonary dysplasia? Cell and Tissue Research 367 (3): 457–468. https://doi.org/10.1007/s00441-016-2534-3.
O'Reilly, M., Thebaud, B. 2014. Animal models of bronchopulmonary dysplasia. The term rat models. Am J Physiol Lung Cell Mol Physiol, 307: L948–58. https://doi.org/10.1152/ajplung. 00160.2014.
Kinsella, J.P., A. Greenough, and S.H. Abman. 2006. Bronchopulmonary dysplasia. Lancet 367: 1421–1431. https://doi.org/10.1016/S0140-6736(06)68615-7.
Negi, R., D. Pande, A. Kumar, R.S. Khanna, and H.D. Khanna. 2012. Evaluation of biomarkers of oxidative stress and antioxidant capacity in the cord blood of preterm low birth weight neonates. The Journal of Maternal-Fetal & Neonatal Medicine 25 (8): 1338–1341. https://doi.org/10.3109/14767058.2011.633672.
Perrone, S., Bracciali, C., Di Virgilio, N., Buonocore, G. 2016. Oxygen use in neonatal care: a two-edged sword. Front Pediatr, 4(undefined), 143. https://doi.org/10.3389/fped.2016.00143.
Ozsurekci, Y., Aykac, K. 2016. Oxidative stress related diseases in newborns. Oxid Med Cell Longev, (undefined), 2768365. https://doi.org/10.1155/2016/2768365.
Balany, J., and V. Bhandari. 2015. Understanding the impact of infection, inflammation, and their persistence in the pathogenesis of bronchopulmonary dysplasia. Frontiers in Medicine 2: 90. https://doi.org/10.3389/fmed.2015.00090.
Rosanna, D.P., and C. Salvatore. 2012. Reactive oxygen species, inflammation, and lung diseases. Current Pharmaceutical Design 18 (26): 3889–3900. https://doi.org/10.2174/138161212802083716.
Wright, C.J., F. Agboke, F. Chen, P. La, G. Yang, and P.A. Dennery. 2010. Nitric oxide inhibits hyperoxia-induced NF-B activation in neonatal pulmonary microvascular endothelial cells. Pediatric Research 68 (6): 484–489. https://doi.org/10.1203/PDR.0b013e3181f917b0.
Zha, L., J. Chen, S. Sun, L. Mao, X. Chu, H. Deng, J. Cai, X. Li, Z. Liu, and W. Cao. 2014. Soyasaponins can blunt inflammation by inhibiting the reactive oxygen species-mediated activation of PI3K/Akt/NF-kB pathway. PLoS ONE 9 (9): e107655. https://doi.org/10.1371/journal.pone.0107655.
Agostini, L., F. Martinon, K. Burns, M.F. McDermott, P.N. Hawkins, and J. Tschopp. 2004. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20 (3): 319–325. https://doi.org/10.1016/s1074-7613(04)00046-9.
Peng, D., J. Li, Y. Deng, X. Zhu, L. Zhao, Y. Zhang, Z. Li, S. Ou, S. Li, and Y. Jiang. 2020. Sodium para-aminosalicylic acid inhibits manganese-induced NLRP3 inflammasome-dependent pyroptosis by inhibiting NF-κB pathway activation and oxidative stress. Journal of Neuroinflammation 17 (1): 343. https://doi.org/10.1186/s12974-020-02018-6.
Teng, J., Mei, Q., Zhou, X., Tang, Y., Xiong, R., Qiu, W., Pan, R., Law Betty, Y., Wong Vincent, K., Yu Chong L. et al. 2020. Polyphyllin VI induces caspase-1-mediated pyroptosis via the induction of ROS/NF-κB/NLRP3/GSDMD signal axis in non-small cell lung cancer. Cancers (Basel), 12(1), undefined. https://doi.org/10.3390/cancers12010193.
Liao, J., V.S. Kapadia, L.S. Brown, N. Cheong, C. Longoria, D. Mija, M. Ramgopal, J. Mirpuri, D.C. McCurnin, and R.C. Savani. 2015. The NLRP3 inflammasome is critically involved in the development of bronchopulmonary dysplasia. Nature Communications 6: 8977. https://doi.org/10.1038/ncomms9977.
Altemimi, A., Lakhssassi, N., Baharlouei, A., Watson, D.G., Lightfoot David, A. 2017. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants (Basel), 6(4), undefined. https://doi.org/10.3390/plants6040042.
El-Saber, B.G., Magdy, B.A., El-Mleeh, A., Abdel-Daim, M.M., Prasad Devkota, H. 2020. Glycyrrhiza glabra Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of L. (Fabaceae). Biomolecules, 10 (3), undefined. https://doi.org/10.3390/biom10030352.
Eichenwald, E.C. 2016. Committee on Fetus and Newborn, American Academy of Pediatrics: Apnea of prematurity. Pediatrics, 137(1), undefined. https://doi.org/10.1542/peds.2015-3757.
Coulter, D.M. 2006. Caffeine for apnea of prematurity. N Engl J Med, 355(9), 958–9; author reply 959–60. https://doi.org/10.1056/NEJMc061615.
Pakvasa, M.A., V. Saroha, and M. Patel Ravi. 2018. Optimizing caffeine use and risk of bronchopulmonary dysplasia in preterm infants: A systematic review, meta-analysis, and application of grading of recommendations assessment, development, and evaluation methodology. Clinics in Perinatology 45 (2): 273–291. https://doi.org/10.1016/j.clp.2018.01.012.
Sanchez-Solis, M., P.W. Garcia-Marcos, J. Agüera-Arenas, P. Mondejar-Lopez, and L. Garcia-Marcos. 2020. Impact of early caffeine therapy in preterm newborns on infant lung function. Pediatric Pulmonology 55 (1): 102–107. https://doi.org/10.1002/ppul.24540.
Liu, Z.X., Xu, S.J., Li, L., Jia, F.F., Guo, H., Chen, Wen-li3, Liu, K., Zhang, Q., Fu, J.H., Liu, J.X. 2021. Analysis of Chinese patent medicines in 2020 Edition of Chinese Pharmacopoeia (Part I) for cough of children. Chinese Journal of Experimental Traditional Medical Formulae, 27:160-167.
Pastorino, G., L. Cornara, S. Soares, F. Rodrigues, and M.B.P.P. Oliveira. 2018. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytotherapy Research 32: 2323–2339. https://doi.org/10.1002/ptr.6178.
Chen, J., W. Zhang, L. Zhang, J. Zhang, X. Chen, M. Yang, T. Chen, and J. Hong. 2017. Glycyrrhetinic acid alleviates radiation-induced lung injury in mice. Journal of Radiation Research 58: 41–47. https://doi.org/10.1093/jrr/rrw091.
Kao, T.C., M.H. Shyu, and G.C. Yen. 2010. Glycyrrhizic acid and 18beta-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3beta signaling and glucocorticoid receptor activation. Journal of Agriculture and Food Chemistry 58: 8623–8629. https://doi.org/10.1021/jf101841r.
Kim, S.H., Hong, J.H., Lee, J.E., Lee, Y.C. 2017. 18β-Glycyrrhetinic acid, the major bioactive component of Glycyrrhizae Radix, attenuates airway inflammation by modulating Th2 cytokines, GATA-3, STAT6, and Foxp3 transcription factors in an asthmatic mouse model. Environmental Toxicology and Pharmacology. Environ Toxicol Pharmacol, 52: 99–113. https://doi.org/10.1016/j.etap.2017.03.011.
Zhang, M., Z. Chang, F. Zhao, P. Zhang, Y.J. Hao, L. Yan, N. Liu, J.L. Wang, L. Bo, P. Ma, W. Zhou, X. Ma, Q.B. Xu, and R. Zhou. 2019. Protective effects of 18β-glycyrrhetinic acid on monocrotaline-induced pulmonary arterial hypertension in rats. Frontiers in Pharmacology 10: 13. https://doi.org/10.3389/fphar.2019.00013.
Cooney, T.P., and W.M. Thurlbeck. 1982. The radial alveolar count method of Emery and Mithal: A reappraisal 2–intrauterine and early postnatal lung growth. Thorax 37: 580–583. https://doi.org/10.1136/thx.37.8.580.
Endesfelder, S., Strauß, E., Bendix, I., Schmitz, T., Bührer, C. 2020. Prevention of oxygen-induced inflammatory lung injury by caffeine in neonatal rats. Oxid Med Cell Longev, (undefined), 3840124. https://doi.org/10.1155/2020/3840124.
Stoll, B.J., N.I. Hansen, E.F. Bell, M.C. Walsh, W.A. Carlo, S. Shankaran, A.R. Laptook, P.J. Sanchez, K.P. Van Meurs, M. Wyckoff, et al. 2015. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 314: 1039–1051. https://doi.org/10.1001/jama.2015.10244.
Stoll, B.J., N.I. Hansen, E.F. Bell, S. Shankaran, A.R. Laptook, M.C. Walsh, E.C. Hale, N.S. Newman, K. Schibler, W.A. Carlo, et al. 2010. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics 126: 443–456. https://doi.org/10.1542/peds.2009-2959.
Endesfelder, S., E. Strauß, T. Scheuer, T. Schmitz, and C. Bührer. 2019. Antioxidative effects of caffeine in a hyperoxia-based rat model of bronchopulmonary dysplasia. Respiratory Research 20 (1): 88. https://doi.org/10.1186/s12931-019-1063-5.
Mizoguchi, K., Ikarashi, Y. 2017. Multiple psychopharmacological effects of the traditional Japanese kampo medicine yokukansan, and the brain regions it affects. Front Pharmacol, 8(undefined), 149. https://doi.org/10.3389/fphar.2017.00149.
Kao, T.C., Wu, C.H., Yen, G.C. 2013. Glycyrrhizic acid and 18β-glycyrrhetinic acid recover glucocorticoid resistance via PI3K-induced AP1, CRE and NFAT activation. Phytomedicine, 20(null), 295–302. https://doi.org/10.1016/j.phymed.2012.10.013.
Akao, T., Akao, T., Hattori, M., Kanaoka, M., Yamamoto, K., Namba, T., Kobashi, K. 1991. Hydrolysis of glycyrrhizin to 18 beta-glycyrrhetyl monoglucuronide by lysosomal beta-D-glucuronidase of animal livers. Biochem Pharmacol, 41(null), 1025–9. https://doi.org/10.1016/0006-2952(91)90210-v.
Kotecha, S. 2000. Lung growth: Implications for the newborn infant. Archives of Disease in Childhood. Fetal and Neonatal Edition 82 (1): F69-74. https://doi.org/10.1136/fn.82.1.f69.
Joshi, S., and S. Kotecha. 2007. Lung growth and development. Early Human Development 83 (12): 789–794. https://doi.org/10.1016/j.earlhumdev.2007.09.007.
Chen, S., Q. Wu, D. Zhong, C. Li, and L. Du. 2020. Caffeine prevents hyperoxia-induced lung injury in neonatal mice through NLRP3 inflammasome and NF-κB pathway. Respiratory Research 21: 140. https://doi.org/10.1186/s12931-020-01403-2.
Ryan, R.M., Q. Ahmed, and S. Lakshminrusimha. 2008. Inflammatory mediators in the immunobiology of bronchopulmonary dysplasia. Clinical Reviews in Allergy and Immunology 34: 174–190. https://doi.org/10.1007/s12016-007-8031-4.
Savani, R.C. 2018. Modulators of inflammation in bronchopulmonary dysplasia.Seminars in Perinatology, 42(7), 459–470. https://doi.org/10.1053/j.semperi.2018.09.009.
Bhatia, M., R.L. Zemans, and S. Jeyaseelan. 2012. Role of chemokines in the pathogenesis of acute lung injury. American Journal of Respiratory Cell and Molecular Biology 46 (5): 566–572. https://doi.org/10.1165/rcmb.2011-0392TR.
Bry, K., J.A. Whitsett, and U. Lappalainen. 2007. IL-1beta disrupts postnatal lung morphogenesis in the mouse. American Journal of Respiratory Cell and Molecular Biology 36: 32–42. https://doi.org/10.1165/rcmb.2006-0116OC.
Bourbia, A., M.A. Cruz, and H.J. Rozycki. 2006. NF-kappaB in tracheal lavage fluid from intubated premature infants: Association with inflammation, oxygen, and outcome. Archives of Disease in Childhood. Fetal and Neonatal Edition 91 (1): F36–F39. https://doi.org/10.1136/adc.2003.045807.
Aghai, Z.H., S. Kumar, S. Farhath, M.A. Kumar, J. Saslow, T. Nakhla, R. Eydelman, L. Strande, G. Stahl, C. Hewitt, M. Nesin, and I. Rahman. 2006. Dexamethasone suppresses expression of nuclear factor-κB in the cells of tracheobronchial lavage fluid in premature neonates with respiratory distress. Pediatric Research 59 (6): 811–815. https://doi.org/10.1203/01.pdr.0000219120.92049.b3.
Aghai, Z.H., A. Kode, J.G. Saslow, T. Nakhla, S. Farhath, G.E. Stahl, R. Eydelman, L. Strande, P. Leone, and I. Rahman. 2007. Azithromycin suppresses activation of nuclear factor-κB and synthesis of pro inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatric Research 62 (4): 483–488. https://doi.org/10.1203/PDR.0b013e318142582d.
Wei, Z., Nie, G., Yang, F., Pi, S., Wang, C., Cao, H., Guo, X., Liu, P., Li, G., Hu, G., Zhang, C. 2020. Inhibition of ROS/NLRP3/Caspase-1 mediated pyroptosis attenuates cadmium-induced apoptosis in duck renal tubular epithelial cells. Environ Pollut, 273(undefined), 115919. https://doi.org/10.1016/j.envpol.2020.115919.
Ishida, T,, Miki, I., Tanahashi, T., Yagi, S., Kondo, Y., Inoue, J., Kawauchi, S., Nishiumi, S., Yoshida, M., Maeda, H., Tode, C., Takeuchi, A., Nakayama, H., Azuma, T., Mizuno, S. 2013. Effect of 18β-glycyrrhetinic acid and hydroxypropyl γcyclodextrin complex on indomethacin-induced small intestinal injury in mice. Eur J Pharmacol, 714(null), 125–31. https://doi.org/10.1016/j.ejphar.2013.06.007.
Yang, Y., Zhu, Q., Zhong, Y., Cui, X., Jiang, Z., Wu, P., Zheng, X., Zhang, K., Zhao, S. 2020. Synthesis, anti-microbial and anti-inflammatory activities of 18β-glycyrrhetinic acid derivatives. Bioorg Chem, 101(undefined), 103985. https://doi.org/10.1016/j.bioorg.2020.103985.
Pagano, C., Calarco, P., Di Michele, A., Ceccarini, M.R., Beccari, T., Primavilla, S., Scuota, S., Marmottini, F., Ramella, D., Ricci, M., Perioli, L. 2021. Development of sodium carboxymethyl cellulose based polymeric microparticles for in situ hydrogel wound dressing formation. Int J Pharm, 602(undefined), 120606. https://doi.org/10.1016/j.ijpharm.2021.120606
Acknowledgements
The authors would like to thank Professor Dongyan Liu from the Department of Immunology, Shengjing Hospital of China Medical University, for technical support.
Funding
This study was supported by grants from the National Natural Science Foundation of China (grant no. 81571479) and the 345 Talent Project of the Shengjing Hospital (grant no. M0428).
Author information
Authors and Affiliations
Contributions
QC, XX, and JF conceived and designed the study. QC, ZL, XZ, and XY performed the experiments. QC, ZL, XZ, and XY analyzed the data. XX and JF reviewed the data. QC wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All animal procedures were approved by the ethics committee of China Medical University (Approval No.: 2021PS267K) and followed institutional guidelines.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qing, C., Ziyun, L., Xuefei, Y. et al. Protective Effects of 18β-Glycyrrhetinic Acid on Neonatal Rats with Hyperoxia Exposure. Inflammation 45, 1224–1238 (2022). https://doi.org/10.1007/s10753-021-01616-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01616-7