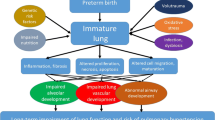
Abstract
Bronchopulmonary dysplasia (BPD) is potentially one of the most devastating conditions in premature infants with longstanding consequences involving multiple organ systems including adverse effects on pulmonary function and neurodevelopmental outcome. Here we review recent studies in the field to summarize the progress made in understanding in the pathophysiology, prognosis, prevention, and treatment of BPD in the last decade. The work reviewed includes the progress in understanding its pathobiology, genomic studies, ventilatory strategies, outcomes, and therapeutic interventions. We expect that this review will help guide clinicians to treat premature infants at risk for BPD better and lead researchers to initiate further studies in the field.

Similar content being viewed by others
References
Northway WH Jr, Rosan RC, Porter DY (1967) Pulmonary disease following respirator therapy of hyaline-membrane disease: bronchopulmonary dysplasia. N Engl J Med 276(7):357–368
Isayama T, Lee SK, Yang J, Lee D, Daspal S, Dunn M, Shah PS (2017) Revisiting the definition of bronchopulmonary dysplasia: effect of changing panoply of respiratory support for preterm neonates. JAMA Pediatr 171(3):271–279
Baraldi E, Filippone M (2007) Chronic lung disease after premature birth. N Engl J Med 357(19):1946–1955
Bhandari V, Bizzarro MJ, Shetty A, Zhong X, Page GP, Zhang H, Ment LR, Gruen JR (2006) Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics 117(6):1901–1906
Birenbaum HJ, Dentry A, Cirelli J, Helou S, Pane MA, Star K, Melick CF, Updegraff L, Arnold C, Tamayo A, Torres V, Gungon N, Liverman S (2009) Reduction in the incidence of chronic lung disease in very low birth weight infants: results of a quality improvement process in a tertiary level neonatal intensive care unit. Pediatrics 123(1):44–50
Kollee L, Cuttini M, Delmas D, Papiernik E, den Ouden A, Agostino R, Boerch K, Bréart G, Chabernaud J, Draper E, Gortner L (2009) Obstetric interventions for babies born before 28 weeks of gestation in Europe: results of the MOSAIC study. BJOG 116(11):1481–1491
Stroustrup A, Trasande L (2010) Epidemiological characteristics and resource use in neonates with bronchopulmonary dysplasia: 1993–2006. Pediatrics 126(2):291–297
Lohmann P, Luna RA, Hollister EB, Devaraj S, Mistretta TA, Welty SE, Versalovic J (2014) The airway microbiome of intubated premature infants: characteristics and changes that predict the development of bronchopulmonary dysplasia. Pediatr Res 76(3):294–301
Lal CV, Travers C, Aghai ZH, Jilling T, Halloran B, Carlo WA, Keeley J, Rezonzew G, Kumar R, Morrow C, Bhandari V, Ambalavanan N (2016) The airway microbiome at birth. Sci Rep 6:31023
Rehan VK, Torday JS (2014) The lung alveolar lipofibroblast: an evolutionary strategy against neonatal hyperoxic lung injury. Antioxid Redox Signal 21(13):1893–1904
Bose C, Marter LJ, Laughon M, O’Shea TM, Allred EN, Karna P, Ehrenkranz RA, Boggess K, Leviton A (2009) Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics 124(3):e450–e458
Ryckman KK, Dagle JM, Kelsey K, Momany AM, Murray JC (2012) Genetic associations of surfactant protein D and angiotensin-converting enzyme with lung disease in preterm neonate. J Perinatol 32(5):349–355
Hadchouel A, Durrmeyer X, Bouzigon E, Incitti R, Huusko J, Jarreau PH, Lenclen R, Demenais F, Franco-Montoya ML, Layouni I, Patkai J (2011) Identification of SPOCK2 as a susceptibility gene for bronchopulmonary dysplasia. Am J Respir Crit Care Med 184(10):1164–1170
Ambalavanan N, Cotten CM, Page GP, Carlo WA, Murray JC, Bhattacharya S, Mariani TJ, Cuna AC, Faye-Petersen OM, Kelly D, Higgins RD (2015) Integrated genomic analyses in bronchopulmonary dysplasia. J Pediatr 166(3):531–537
Lavoie PM, Pham C, Jang KL (2008) Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics 122(3):479–485
Been JV, Rours IG, Kornelisse RF, Jonkers F, Krijger RRd, Zimmermann LJ (2010) Chorioamnionitis alters the response to surfactant in preterm infants. J Pediatr 156(1):10–15
de Haan TR, Beckers L, de Jonge RC, Spanjaard L, Toledo L, Pajkrt D, van Wassenaer-Leemhuis AG, van der Lee JH (2013) Neonatal gram negative and candida sepsis survival and neurodevelopmental outcome at the corrected age of 24 months. PLoS ONE 8(3):e59214
Eriksson L, Haglund B, Odlind V, Altman M, Ewald U, Kieler H (2015) Perinatal conditions related to growth restriction and inflammation are associated with an increased risk of bronchopulmonary dysplasia. Acta Paediatr 104(3):259–263
Lowe J, Watkins WJ, Edwards MO, Spiller OB, Jacqz-Aigrain E, Kotecha SJ, Kotecha S (2014) Association between pulmonary ureaplasma colonization and bronchopulmonary dysplasia in preterm infants: updated systematic review and meta-analysis. Pediatr Infect Dis J 33(7):697–702
Collard KJ (2006) Is there a causal relationship between the receipt of blood transfusions and the development of chronic lung disease of prematurity? Med Hypotheses 66(2):355–364
Valieva OA, Strandjord TP, Mayock DE, Juul SE (2009) Effects of transfusions in extremely low birth weight infants: a retrospective study. J Pediatr 155(3):331–337
Fakhoury KF, Sellers C, Smith E, Rama JA, Fan LL (2010) Serial measurements of lung function in a cohort of young children with bronchopulmonary dysplasia. Pediatrics 125(6):e1441–e1447
Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, Thomas S, Stocks J (2010) Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med 182(2):237–245
Davidson LM, Berkelhamer SK (2017) Bronchopulmonary dysplasia: chronic lung disease of infancy and long-term pulmonary outcomes. J Clin Med 6(1):4
Trittmann JK, Nelin LD, Klebanoff MA (2013) Bronchopulmonary dysplasia and neurodevelopmental outcome in extremely preterm neonates. Eur J Pediatr 172(9):1173–1180
Bui CB, Pang MA, Sehgal A, Theda C, Lao JC, Berger PJ, Nold MF, Nold-Petry CA (2017) Pulmonary hypertension associated with bronchopulmonary dysplasia in preterm infants. J Reprod Immunol 124:21–29
Levy PT, Dioneda B, Holland MR, Sekarski TJ, Lee CK, Mathur A, Cade WT, Cahill AG, Hamvas A, Singh GK (2015) Right ventricular function in preterm and term neonates: reference values for right ventricle areas and fractional area of change. J Am Soc Echocardiogr 28(5):559–569
Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, Stenmark KR, Steinhorn R, Thébaud B, Fineman JR, Kuehne T, Feinstein JA, Kuehne T, Feinstein JA, Friedberg MK, Earing M, Barst RJ, Keller RL, Kinsella JP, Mullen M, Deterding R, Kulik T, Mallory G, Humpl T, Wessel DL (2015) Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation 132(21):2037–2099
Ballard HO, Anstead MI, Shook LA (2007) Azithromycin in the extremely low birth weight infant for the prevention of bronchopulmonary dysplasia: a pilot study. Respir Res 8(1) 41
Ballard HO, Shook LA, Bernard P, Anstead MI, Kuhn R, Whitehead V, Grider D, Crawford TN, Hayes D (2011) Use of azithromycin for the prevention of bronchopulmonary dysplasia in preterm infants: a randomized, double-blind, placebo controlled trial. Pediatr Pulmonol 46(2):111–118
Mandell E, Seedorf G, Gien J, Abman SH 2014() Vitamin D treatment improves survival and infant lung structure after intra-amniotic endotoxin exposure in rats: potential role for the prevention of bronchopulmonary dysplasia. Am J Physiol-Lung Cell Mol Physiol 306(5):L420–L428
Nold MF, Mangan NE, Rudloff I, Cho SX, Shariatian N, Samarasinghe TD, Skuza EM, Pedersen J, Veldman A, Berger PJ, Nold-Petry CA (2013) Interleukin-1 receptor antagonist prevents murine bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Proc Natl Acad Sci USA 110(35):14384–14389
Doyle LW, Ehrenkranz RA, Halliday HL (2014) Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev 5:CD001146
Doyle LW, Ehrenkranz RA, Halliday HL (2014) Late (> 7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database Syst Rev 5:CD001145
Onland W, Offringa M, Jaegere APD, van Kaam AH (2009) Finding the optimal postnatal dexamethasone regimen for preterm infants at risk of bronchopulmonary dysplasia: a systematic review of placebo-controlled trials. Pediatrics 123(1):367–377
Parikh NA, Lasky RE, Kennedy KA, Moya FR, Hochhauser L, Romo S, Tyson JE (2007) Postnatal dexamethasone therapy and cerebral tissue volumes in extremely low birth weight infants. Pediatrics 119(2):265–272
Yeh TF, Lin HC, Chang CH, Wu TS, Su BH, Li TC, Pyati S, Tsai CH (2008) Early intratracheal instillation of budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants: a pilot study. Pediatrics 121(5):e1310–e1318
Venkataraman R, Kamaluddeen M, Hasan SU, Robertson HL, Lodha A (2017) Intratracheal administration of budesonide-surfactant in prevention of bronchopulmonary dysplasia in very low birth weight infants: a systematic review and meta-analysis. Pediatr Pulmonol 52(7):968–975
Baud O, Maury L, Lebail F, Ramful D, Moussawi FE, Nicaise C, Zupan-Simunek V, Coursol A, Beuchée A, Bolot P, Andrini P, Mohamed D, Alberti C (2016) Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. Lancet 387(10030):1827–1836
Baud O, Trousson C, Biran V, Leroy E, Mohamed D, Alberti C (2017) association between early low-dose hydrocortisone therapy in extremely preterm neonates and neurodevelopmental outcomes at 2 years of age. JAMA 317(13):1329–1337
Fischer HS, Bührer C (2013) Avoiding endotracheal ventilation to prevent bronchopulmonary dysplasia: a meta-analysis. Pediatrics 132(5):e1351–e1360
Biniwale M, Wertheimer F (2017) Decrease in delivery room intubation rates after use of nasal intermittent positive pressure ventilation in the delivery room for resuscitation of very low birth weight infants. Resuscitation 116:33–38
Kugelman A, Feferkorn I, Riskin A, Chistyakov I, Kaufman B, Bader D (2007) Nasal intermittent mandatory ventilation versus nasal continuous positive airway pressure for respiratory distress syndrome: a randomized, controlled, prospective study. J Pediatr 150(5):521–526
Wheeler KI, Klingenberg C, Morley CJ, Davis PG (2011) Volume-targeted versus pressure-limited ventilation for preterm infants: a systematic review and meta-analysis. Neonatology 100(3):219–227
Bhandari V, Finer NN, Ehrenkranz RA, Saha S, Das A, Walsh MC, Engle WA, VanMeurs KP (2009) Synchronized nasal intermittent positive-pressure ventilation and neonatal outcomes. Pediatrics 124(2):517–526
SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network (2010) Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med 362(21):1959–1969
Askie LM, Henderson-Smart DJ, Irwig L, Simpson JM (2003) Oxygen-saturation targets and outcomes in extremely preterm infants. N Engl J Med 349(10):959–967
Saugstad OD, Aune D (2013) Optimal oxygenation of extremely low birth weight infants: a meta-analysis and systematic review of the oxygen saturation target studies. Neonatology 105(1):55–63
Rojas MA, Lozano JM, Rojas MX, Laughon M, Bose CL, Rondon MA, Charry L, Bastidas JA, Perez LA, Rojas C, Ovalle O, Celis LA, Garcia-Harker J, Jaramillo ML (2009) Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: a randomized, controlled trial. Pediatrics 123(1):137–142
Laughon M, Bose C, Moya F, Aschner J, Donn SM, Morabito C, Cummings JJ, Segal R, Guardia C, Liu G (2009) A pilot randomized, controlled trial of later treatment with a peptide-containing, synthetic surfactant for the prevention of bronchopulmonary dysplasia. Pediatrics 123(1):89–96
Stevens TP, Blennow M, Myers EH, Soll R (2007) Early surfactant administration with brief ventilation versus selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev 4:CD003063
Rojas-Reyes MX, Morley CJ, Soll R (2012) Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 3:CD000510
Lopez E, Gascoin G, Flamant C, Merhi M, Tourneux P, Baud O (2013) Exogenous surfactant therapy in 2013: what is next? Who, when and how should we treat newborn infants in the future? BMC Pediatr 13(1):165
Sato A, Ikegami M (2012) SP-B and containing SP-C new synthetic surfactant for treatment of extremely immature lamb lung. PLoS ONE 7(7):e39392
Van Meurs KP, Wright LL, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, Perritt R, Higgins RD, Oh W, Hudak ML, Laptook AR, Shankaran S, Finer NN, Carlo WA, Kennedy KA, Fridriksson JH, Steinhorn RH, Sokol GM, Konduri G, Aschner JL, Stoll BJ, D’Angio CT, Stevenson DK (2005) Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med 353(1):13–22
Cole FS, Alleyne C, Barks JD, Boyle RJ, Carroll JL, Dokken D, Edwards WH, Georgieff M, Gregory K, Johnston MV, Kramer M, Mitchell C, Neu J, Pursley DM, Robinson WM, Rowitch DH (2011) NIH consensus development conference: inhaled nitric oxide therapy for premature infants. NIH consensus and state-of-the-science statements. Pediatrics 127(2):363–369
Hibbs AM, Walsh MC, Martin RJ, Truog WE, Lorch SA, Alessandrini E, Cnaan A, Palermo L, Wadlinger SR, Coburn CE, Ballard PL, Ballard RA (2008) One-year respiratory outcomes of preterm infants enrolled in the nitric oxide (to prevent) chronic lung disease trial. J Pediatr 153(4):525–529
Ellsworth MA, Harris MN, Carey WA, Spitzer AR, Clark RH (2015) Off-label use of inhaled nitric oxide after release of NIH consensus statement. Pediatrics 135(4):643–648
Clyman R, Cassady G, Kirklin JK, Collins M, Philips JB (2009) The role of patent ductus arteriosus ligation in bronchopulmonary dysplasia: reexamining a randomized controlled trial. J Pediatr 154(6):873–876
Youn Y, Lee J-Y, Lee JH, Kim S-Y, Sung IK, Lee JY (2013) Impact of patient selection on outcomes of PDA in very low birth weight infants. Early Hum Develop 89(3):175–179
Jhaveri N, Moon-Grady A, Clyman RI (2010) Early surgical ligation versus a conservative approach for management of patent ductus arteriosus that fails to close after indomethacin treatment. J Pediatr 157(3):381–387
Sung SI, Chang YS, Chun JY, Yoon SA, Yoo HS, Ahn SY, Park WS (2016) Mandatory closure versus nonintervention for patent ductus arteriosus in very preterm infants. J Pediatr 182:66–71
Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, Stoll BJ, Stevenson JALK, Bauer CR, Korones SB, Donovan EF, Carlo WA, Shankaran S, Stark AR, Papile L-A, Jobe A, Stacewicz-Sapuntzakis M, Verter J, Fanaroff AA (1999) Vitamin A supplementation for extremely-low-birth-weight infants. N Engl J Med 340(25):1962–1968
Darlow BA, Graham PJ (2011) Vitamin A supplementation to prevent mortality and short and long-term morbidity in very low birthweight infants. Cochrane Database Syst Rev 10:CD000501
Ambalavanan N, Tyson JE, Kennedy KA, Hansen NI, Vohr BR, Wright LL, Carlo WA (2005) Vitamin A supplementation for extremely low birth weight infants: outcome at 18 to 22 months. Pediatrics 115(3):e249–e254,
Uberos J, Miras-Baldo M, Jerez-Calero A, Narbona-López E (2014) Effectiveness of vitamin A in the prevention of complications of prematurity. Pediatrics & Neonatology 55(5):358–362
Gadhia MM, Cutter GR, Abman SH, Kinsella JP (2014) Effects of early inhaled nitric oxide therapy and vitamin A supplementation on the risk for bronchopulmonary dysplasia in premature newborns with respiratory failure. J Pediatr 164(4):744–748
Dobson NR, Patel RM, Smith PB, Kuehn DR, Clark J, Vyas-Read S, Herring A, Laughon MM, Carlton D, Hunt CE (2014) Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants. J Pediatr 164(5):992–998
Dekker J, Hooper SB, Vonderen JJv, Witlox RS, Lopriore E, te Pas AB (2017) Caffeine to improve breathing effort of preterm infants at birth: a randomized controlled trial. Pediatr Res 82:290–296
Valdez RC, Ahlawat R, Wills-Karp M, Nathan A, Ezell T, Gauda EB (2011) Correlation between serum caffeine levels and changes in cytokine profile in a cohort of preterm infants. J Pediatr 158(1):57–64
Stewart A, Brion LP (2011) Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database of Syst Rev 9:CD001453
Pierro M, Thébaud B, Soll R (2017) Mesenchymal stem cells for the prevention and treatment of bronchopulmonary dysplasia in preterm infants. Cochrane Database of Syst Rev 11:CD011932
O’Reilly M, Thébaud B (2013) Using cell-based strategies to break the link between bronchopulmonary dysplasia and the development of chronic lung disease in later. Pulm Med 2013:874161
Fung ME, Thébaud B (2013) Stem cell-based therapy for neonatal lung disease: it is in the juice. Pediatr Res 75(1):2–7
Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA (2011) Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells 29(6):913–919
Chang YS, Ahn SY, Yoo HS, Sung SI, Choi SJ, Oh WI, Park WS (2014) Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr 164(5):966–972
Sakurai R, Shin E, Fonseca S, Sakurai T, Litonjua AA, Weiss ST, Torday JS, Rehan VK (2009) 1α, 25 (OH) 2D3 and its 3-epimer promote rat lung alveolar epithelial-mesenchymal interactions and inhibit lipofibroblast apoptosis. Am J Physiol Lung Cell Mol Physiol 297(3):L496–L505
Yurt M, Liu J, Sakurai R, Gong M, Husain SM, Siddiqui MA, Husain M, Villarreal P, Akcay F, Torday JS, Rehan VK (2014) Vitamin D supplementation blocks pulmonary structural and functional changes in a rat model of perinatal vitamin D deficiency. Am J Physiol Lung Cell Mol Physiol 307(11):L859–L867
Cerny L, Torday JS, Rehan VK (2008) Prevention and treatment of bronchopulmonary dysplasia: contemporary status and future outlook. Lung 186(2):75–89
Rehan VK, Torday JS (2012) PPARγ signaling mediates the evolution, development, homeostasis, and repair of the lung. PPAR Res 2012:289867
De Visser YP, Walther FJ, Laghmani EH, Boersma H, Laarse AVd, Wagenaar GT (2009) Sildenafil attenuates pulmonary inflammation and fibrin deposition, mortality and right ventricular hypertrophy in neonatal hyperoxic lung injury. Respir Res 10(1):30
Tan K, Krishnamurthy MB, O’Heney JL, Paul E, Sehgal A (2015) Sildenafil therapy in bronchopulmonary dysplasia-associated pulmonary hypertension: a retrospective study of efficacy and safety. Eur J Pediatr 174(8):1109–1115
Wolfson MR, Funanage VL, Kirwin SM, Pilon AL, Shashikant BN, Miller TL, Shaffer TH (2008) Recombinant human Clara cell secretory protein treatment increases lung mRNA expression of surfactant proteins and vascular endothelial growth factor in a premature lamb model of respiratory distress syndrome. Am J Perinatol 25(10):637–645
Miller TL, Shashikant BN, Melby JM, Pilon AL, Shaffer TH, Wolfson MR (2005) Recombinant human Clara cell secretory protein in acute lung injury of the rabbit: effect of route of administration. Pediatr Crit Care Med 6(6):698–706
Davis J, Parad R (2013) Safety, pharmacokinetics, and anti-inflammatory effects of intratracheal recombinant human Clara cell protein in premature infants with respiratory distress syndrome. ClinicalTrials.gov Identifier NCT01941745
Taylor S, Rehan VK (2016) Anti-inflammatory agents for the prevention of bronchopulmonary dysplasia. In: Bronchopulmonary dysplasia. Springer, New York, pp 325–344
Funding
Funding for the study was provided by NIH (HD51857, HL107118, HD071731, and HL127237) and TRDRP (17RT-0170 and 23RT-0018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Jung Hwang and Dr. Virender Rehan declare that they have no conflict of interest.
Research Involving Human Participants and/or Animal
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed for the animal studies performed by the authors. This chapter does not contain any studies with human participants performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Hwang, J.S., Rehan, V.K. Recent Advances in Bronchopulmonary Dysplasia: Pathophysiology, Prevention, and Treatment. Lung 196, 129–138 (2018). https://doi.org/10.1007/s00408-018-0084-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-018-0084-z