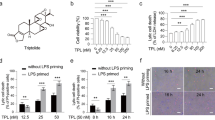
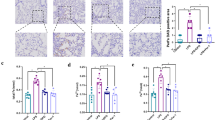
Abstract
We have previously shown that diallyl disulfide (DADS) protects mice against cerulein-induced acute pancreatitis (AP) and associated lung injury. However, the molecular mechanisms underlying its effect and the components involved have not been studied. We hypothesized that DADS may reduce TNF-α, CSE expression, H2S production, STAT3, and NF-κB activation and induce SOCS3 expression through peroxisome proliferator-activated receptor γ (PPAR-γ) pathway in cerulein-induced mice. Male Swiss mice were treated with hourly intraperitoneal injections of cerulein (50 µg/kg) for 6 h. Diallyl disulfide (200 μg/kg) was administered in the presence or absence of PPAR-γ antagonist GW9662 (0.3 mg/kg) (i.p) 1 h after the induction of AP. Our findings revealed that DADS blocked TNF-α, CSE expression, H2S production, and STAT3, and NF-κB activation was reversed by GW9662. Furthermore, GW9662 abrogated DADS-induced SOCS3 expression. The results show for the first that DADS-induced anti-inflammatory effect in acute pancreatitis is regulated through PPAR-γ.








Similar content being viewed by others
Data Availability
Data sharing is not applicable to this article and no datasets were generated.
References
Shah, S.S.R., and Jimil. 2006. Acute respiratory distress syndrome in acute pancreatitis, Immunopathologia Persa. 6. https://doi.org/10.34172/ipp.2020.16.
Wu, J., L. Zhang, J. Shi, R. He, W. Yang, A. Habtezion, N. Niu, P. Lu, and J. Xue. 2020. Macrophage phenotypic switch orchestrates the inflammation and repair/regeneration following acute pancreatitis injury. eBioMedicine 58: 102920. https://doi.org/10.1016/j.ebiom.2020.102920.
Zhang, H., W. Yang, Y. Li, L. Hu, Y. Dai, J. Chen, S. Xu, X. Xu, and H. Jiang. 2018. Astaxanthin ameliorates cerulein-induced acute pancreatitis in mice. International Immunopharmacology. 56: 18–28. https://doi.org/10.1016/j.intimp.2018.01.011.
Pendharkar, S.A., R.G. Singh, S.K. Chand, A. Cervantes, and M.S. Petrov. 2018. Pro-inflammatory cytokines after an episode of acute pancreatitis: Associations with fasting gut hormone profile. Inflammation Research. 67: 339–350. https://doi.org/10.1007/s00011-017-1125-4.
Jakkampudi, A., R. Jangala, R. Reddy, S. Mitnala, G.V. Rao, R. Pradeep, D.N. Reddy, and R. Talukdar. 2017. Acinar injury and early cytokine response in human acute biliary pancreatitis. Scientific Reports. 7: 1–13. https://doi.org/10.1038/s41598-017-15479-2.
Ang, A.D., J. Rivers-Auty, A. Hegde, I. Ishii, and M. Bhatia. 2013. The effect of CSE gene deletion in caerulein-induced acute pancreatitis in the mouse. AJP: Gastrointestinal and Liver Physiology. 305: G712–G721. https://doi.org/10.1152/ajpgi.00044.2013.
Gaddam, R.R., R. Fraser, A. Badiei, S. Chambers, V.C. Cogger, D.G. Le Couteur, I. Ishii, and M. Bhatia. 2016. Cystathionine-gamma-lyase gene deletion protects mice against inflammation and liver sieve injury following polymicrobial sepsis. PLoS ONE 11: 1–21. https://doi.org/10.1371/journal.pone.0160521.
Jin, W., and Y. Shen. 2019. Decoction alleviates intestinal injury in rats with severe acute pancreatitis by inhibiting the JAK2-STAT3 signaling pathway. Evidence-Based Complementary and Alternative Medicine. 2019. https://doi.org/10.1155/2019/3909468.
Robinson, K., L. Vona-Davis, D. Riggs, B. Jackson, and D. McFadden. 2006. Peptide YY attenuates STAT1 and STAT3 activation induced by TNF-α in acinar cell line AR42J. Journal of the American College of Surgeons. 202: 788–796. https://doi.org/10.1016/j.jamcollsurg.2006.01.007.
Watanabe, T., M. Kudo, and W. Strober. 2017. Immunopathogenesis of pancreatitis. Mucosal Immunology. 10: 283–298. https://doi.org/10.1038/mi.2016.101.
Szatmary, P., and I. Gukovsky. 2016. Angeles, The role of cytokines and inflammation in the genesis of experimental cell recruitment 3 Chemokines which recruit innate immune cells in pancreatitis. Pancreapedia: The Exocrine Pancreas Knowledge Base. 1. https://doi.org/10.3998/PANC.2016.29.
G. 2019. Access, Cystathionine γ lyase – hydrogen sulfide increases peroxisome proliferator-activated receptor γ activity by sulfhydration at C139 site thereby promoting glucose uptake and lipid storage in adipocytes. 2–3.
Rao, C.Y., L.Y. Fu, C.L. Hu, D.X. Chen, T. Gan, Y.C. Wang, and X.Y. Zhao. 2015. H2S mitigates severe acute pancreatitis through the PI3K/AKT-NF-kappaB pathway in vivo. World Journal of Gastroenterology. 21: 4555–4563. https://doi.org/10.3748/wjg.v21.i15.4555.
Tamizhselvi, R., Y.H. Koh, J. Sun, H. Zhang, and M. Bhatia. 2010. Hydrogen sulfide induces ICAM-1 expression and neutrophil adhesion to caerulein-treated pancreatic acinar cells through NF-κB and Src-family kinases pathway. Experimental Cell Research. 316: 1625–1636. https://doi.org/10.1016/j.yexcr.2010.02.044.
Xu, P., X.L. Lou, and C. Chen. 2016. Apoptotic mechanisms of peroxisome proliferator-activated receptor-γ activation in acinar cells during acute pancreatitis. Pancreas 45: 179–186. https://doi.org/10.1097/MPA.0000000000000495.
Heming, M., S. Gran, S.L. Jauch, L. Fischer-Riepe, A. Russo, L. Klotz, S. Hermann, M. Schäfers, J. Roth, and K. Barczyk-Kahlert. 2018. Peroxisome proliferator-activated receptor-γ modulates the response of macrophages to lipopolysaccharide and glucocorticoids Frontiers in Immunology. 9. https://doi.org/10.3389/fimmu.2018.00893.
Eun Ah Song, and H.K. Joo Weon Lim. 2017. Docosahexaenoic acid inhibits IL-6 expression via PPARγ-mediated.pdf. 1357–2725.
Yu, W.G., G. Xu, G.J. Ren, X. Xu, H.Q. Yuan, X.L. Qi, and K.L. Tian. 2011. Preventive action of curcumin in experimental acute pancreatitis in mouse. Indian Journal of Medical Research. 134: 717–724.
Scirpo, R., R. Fiorotto, A. Villani, M. Amenduni, C. Spirili, and M. Strazzabosco. 2016. Stimulation of nuclear receptor PPAR-γ limits NF-kB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology 62: 1551–1562. https://doi.org/10.1002/hep.28000.Stimulation.
Feng, P. Y., Xu, B. Tong, X. Tong, Y. Bian, S. Zhao, and H. Shen. 2019. Saikosaponin a attenuates hyperlipidemic pancreatitis in rats via the PPAR‑γ/NF‑κB signaling pathway. Experimental and Therapeutic Medicine. 1203–1212. https://doi.org/10.3892/etm.2019.8324.
Qin, M.Z., M.B. Qin, Z.H. Liang, and G.D. Tang. 2019. Effect of SOCS3 on lung injury in rats with severe acute pancreatitis through regulating JAK2/STAT3 signaling pathway. European Review for Medical and Pharmacological Sciences. 23: 10123–10131. https://doi.org/10.26355/eurrev_201911_19582.
Jakkampudi, A., R. Jangala, B.R. Reddy, S. Mitnala, D.N. Reddy, and R. Talukdar. 2016. NF-κB in acute pancreatitis: Mechanisms and therapeutic potential. Pancreatology 16: 477–488. https://doi.org/10.1016/j.pan.2016.05.001.
Chen, P., L. Huang, Y. Zhang, M. Qiao, W. Yao, and Y. Yuan. 2011. The antagonist of the JAK-1/STAT-1 signaling pathway improves the severity of cerulein-stimulated pancreatic injury via inhibition of NF-??B activity. International Journal of Molecular Medicine. 27: 731–738. https://doi.org/10.3892/ijmm.2011.632.
Huang, H., Y. Liu, J. Daniluk, S. Gaiser, J. Chu, H. Wang, Z.S. Li, C.D. Logsdon, and B. Ji. 2013. Activation of nuclear factor-κB in acinar cells increases the severity of pancreatitis in mice. Gastroenterology 144: 202–210. https://doi.org/10.1053/j.gastro.2012.09.059.
Chen, W.D., J.L. Zhang, X.Y. Wang, Z.W. Hu, and Y.B. Qian. 2019. The JAK2/STAT3 signaling pathway is required for inflammation and cell death induced by cerulein in AR42J cells. European Review for Medical and Pharmacological Sciences. 23: 1770–1777. https://doi.org/10.26355/eurrev_201902_17139.
Li, M., X. Zhang, B. Wang, X. Xu, X. Wu, M. Guo, and F. Wang. 2018. Effect of JAK2/STAT3 signaling pathway on liver injury associated with severe acute pancreatitis in rats. Experimental and Therapeutic Medicine. 16: 2013–2021. https://doi.org/10.3892/etm.2018.6433.
Wang, Q., L. Bai, S. Luo, T. Wang, F. Yang, J. Xia, H. Wang, K. Ma, M. Liu, S. Wu, H. Wang, S. Guo, X. Sun, and Q. Xiao. 2020. TMEM16A Ca2+-activated Cl− channel inhibition ameliorates acute pancreatitis via the IP3R/Ca2+/NFκB/IL-6 signaling pathway. Journal of Advanced Research. 23: 25–35. https://doi.org/10.1016/j.jare.2020.01.006.
Ji, Z., L. He, A. Regev, and K. Struhl. 2019. Inflammatory regulatory network mediated by the joint action of NF-kB, STAT3, and AP-1 factors is involved in many human cancers. Proceedings of the National Academy of Sciences of the United States of America. 116: 9453–9462. https://doi.org/10.1073/pnas.1821068116.
Végran, F., H. Berger, F. Ghiringhelli, and L. Apetoh. 2013. Socs3 induction by PPARγ restrains cancer-promoting inflammation. Cell Cycle 12: 2157–2158. https://doi.org/10.4161/cc.25370.
You, J., X. Shi, H. Liang, J. Ye, L. Wang, H. Han, H. Fang, W. Kang, and T. Wang. 2017. Cystathionine- Y-lyase promotes process of breast cancer in association with STAT3 signaling pathway. Oncotarget. 8: 65677–65686. https://doi.org/10.18632/oncotarget.20057.
Boumitri, C., E. Brown, and M. Kahaleh. 2017. Necrotizing pancreatitis: Current management and therapies. Clinical Endoscopy. 50: 357–365. https://doi.org/10.5946/ce.2016.152.
Gaman, L., D. Dragos, A. Vlad, G.C. Robu, M.P. Radoi, L. Stroica, M. Badea, and M. Gilca. 2018. Phytoceuticals in acute pancreatitis: targeting the balance between apoptosis and necrosis. Evidence-Based Complementary and Alternative Medicine. 2018. https://doi.org/10.1155/2018/5264592.
Mathan Kumar, M., and R. Tamizhselvi. 2020. Protective effect of diallyl disulfide against cerulein-induced acute pancreatitis and associated lung injury in mice. International Immunopharmacology. 80. https://doi.org/10.1016/j.intimp.2019.106136.
Wang, G., G. Liu, Y. Ye, Y. Fu, and X. Zhang. 2016. Upregulation of miR-34a by diallyl disulfide suppresses invasion and induces apoptosis in SGC-7901 cells through inhibition of the PI3K/Akt signaling pathway. Oncology Letters. 11: 2661–2667. https://doi.org/10.3892/ol.2016.4266.
Bauer, D., N. Redmon, E. Mazzio, E. Taka, J.S. Reuben, A. Day, S. Sadrud-Din, H. Flores-Rozas, K.F.A. Soliman, and S. Darling-Reed. 2015. Diallyl disulfide inhibits TNFα induced CCL2 release through MAPK/ERK and NF-Kappa-B signaling. Cytokine 75: 117–126. https://doi.org/10.1016/j.cyto.2014.12.007.
Yang, J., R. Tang, J. Yi, Y. Chen, X. Li, T. Yu, and J. Fei. 2019. Diallyl disulfide alleviates inflammatory osteolysis by suppressing osteoclastogenesis via NF-κB–NFATc1 signal pathway. FASEB Journal. 33: 7261–7273. https://doi.org/10.1096/fj.201802172R.
Khatua, T.N., A.K. Dinda, U.K. Putcha, and S.K. Banerjee. 2016. Diallyl disulfide ameliorates isoproterenol induced cardiac hypertrophy activating mitochondrial biogenesis via eNOS-Nrf2-Tfam pathway in rats. Biochemistry and Biophysics Reports. 5: 77–88. https://doi.org/10.1016/j.bbrep.2015.11.008.
Ko, J.W., S.H. Jeong, H.J. Kwon, N.R. Shin, Y.S. Seo, J.C. Kim, I.S. Shin, and J.S. Kim. 2018. Preventive effect of garlic oil and its organosulfur component diallyl-disulfide on cigarette smoke-induced airway inflammation in mice. Nutrients 10: 1–12. https://doi.org/10.3390/nu10111659.
Ju, K.D., J.W. Lim, and H. Kim. 2017. Peroxisome proliferator-activated receptor-gamma inhibits the activation of STAT3 in cerulein-stimulated pancreatic acinar cells. 22: 189–194.
Pagliei, B., K. Aquilano, S. Baldelli, and M.R. Ciriolo. 2013. Garlic-derived diallyl disulfide modulates peroxisome proliferator activated receptor gamma co-activator 1 alpha in neuroblastoma cells. Biochemical Pharmacology. 85: 335–344. https://doi.org/10.1016/j.bcp.2012.11.007.
Leema, G., and R. Tamizhselvi. 2018. Protective effect of scopoletin against cerulein-induced acute pancreatitis and associated lung injury in mice. Pancreas. 47: 577–585. https://doi.org/10.1097/MPA.0000000000001034.
Mathan Kumar, and M. R. Tamizhselvi. 2020. Protective effect of diallyl disulfide against cerulein-induced acute pancreatitis and associated lung injury in mice. International Immunopharmacology. 80: 106136. https://doi.org/10.1016/j.intimp.2019.106136.
Zhao, C., S. Duquet, and Y.X. Zhou. 1998. Effects of combined use of diallyl disulfide and N-acetyl-cysteine on acetaminophen hepatotoxicity in β-naphthoflavone-pretreated mice. World Journal of Gastroenterology. 4: 112–116.
Xie, H., M. Yang, B. Zhang, M. Liu, and S. Han. 2017. Protective role of TNIP2 in myocardial injury induced by acute pancreatitis and its mechanism. Medical Science Monitor. 23: 5650–5656. https://doi.org/10.12659/MSM.904398.
Yu, W.G., G. Xu, G.J. Ren, X. Xu, H.Q. Yuan, X.L. Qi, and K.L. Tian. 2011. Preventive action of curcumin in experimental acute pancreatitis in mouse. Indian Journal of Medical Research. 134: 717–724. https://doi.org/10.4103/0971-5916.91009.
Koh, Y.H., R. Tamizhselvi, S. Moochhala, J.S. Bian, and M. Bhatia. 2011. Role of protein kinase C in caerulein induced expression of substance P and neurokinin-1-receptors in murine pancreatic acinar cells. Journal of Cellular and Molecular Medicine. 15: 2139–2149. https://doi.org/10.1111/j.1582-4934.2010.01205.x.
Park, H.Y., N.D. Kim, G.Y. Kim, H.J. Hwang, B.W. Kim, W.J. Kim, and Y.H. Choi. 2012. Inhibitory effects of diallyl disulfide on the production of inflammatory mediators and cytokines in lipopolysaccharide-activated BV2 microglia. Toxicology and Applied Pharmacology. 262: 177–184. https://doi.org/10.1016/j.taap.2012.04.034.
de Jong, P.R., A.W.L. Schadenberg, T. van den Broek, J.M. Beekman, F. van Wijk, P.J. Coffer, B.J. Prakken, and N.J.G. Jansen. 2012. STAT3 regulates monocyte TNF-alpha production in systemic inflammation caused by cardiac surgery with cardiopulmonary bypass. PLoS ONE 7: 1–9. https://doi.org/10.1371/journal.pone.0035070.
Fouad, A.A., H.M. Hafez, and A.A.H. Hamouda. 2020. Hydrogen sulfide modulates IL-6/STAT3 pathway and inhibits oxidative stress, inflammation, and apoptosis in rat model of methotrexate hepatotoxicity. Human and Experimental Toxicology. 39: 77–85. https://doi.org/10.1177/0960327119877437.
Gao, Y., H. Zhao, P. Wang, J. Wang, and L. Zou. 2018. The roles of SOCS3 and STAT3 in bacterial infection and inflammatory diseases. Scandinavian Journal of Immunology. 88: 1–12. https://doi.org/10.1111/sji.12727.
Yu, J.H., and H. Kim. 2012. Role of janus kinase/signal transducers and activators of transcription in the pathogenesis of pancreatitis and pancreatic cancer. Gut and Liver. 6: 417–422. https://doi.org/10.5009/gnl.2012.6.4.417.
Croasdell, A., P.F. Duffney, N. Kim, S.H. Lacy, P.J. Sime, and R.P. Phipps. 2015. PPAR γ and the innate immune system mediate the resolution of inflammation. PPAR Research. 2015. https://doi.org/10.1155/2015/549691.
Rivers, J.R., A. Badiei, and M. Bhatia. 2012. Hydrogen sulfide as a therapeutic target for inflammation. Expert Opinion on Therapeutic Targets. 16: 439–449. https://doi.org/10.1517/14728222.2012.673591.
Ding, J.-L., Z.-G. Zhou, X.-Y. Zhou, B. Zhou, L. Wang, R. Wang, L. Zhan, X.-F. Sun, and Y. Li. 2013. Attenuation of acute pancreatitis by peroxisome proliferator-activated receptor-α in rats. Pancreas 42: 114–122. https://doi.org/10.1097/mpa.0b013e3182550cc4.
Acknowledgements
This work was supported by Seed Grant from the Vellore Institute of Technology and Technical supported by the Technical Business Incubator of Vellore Institute of Technology, Vellore, Tamil Nadu, India.
Author information
Authors and Affiliations
Contributions
Category 1 Conception and design of study: Dr. R. Tamizhselvi, Acquisition of data: M. Mathan Kumar, Analysis and or interpretation of data: M. Mathan Kumar, Dr. M. Anbalagan, Dr. R. Tamizhselvi, Category 2, Drafting the manuscript: M. Mathan Kumar, Dr. R. Tamizhselvi, Revising the manuscript critically for important intellectual content: Dr. R. Tamizhselvi, Category 3, Approval of the version of the manuscript to be published: M. Mathan Kumar, Dr. R. Tamizhselvi.
Corresponding author
Ethics declarations
Ethics Approval
Animal ethical approved by Institutional animal ethical committee (Approval No.VIT/IAEC/13/Feb13/22).
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marimuthu, M.K., Moorthy, A. & Ramasamy, T. Diallyl Disulfide Attenuates STAT3 and NF-κB Pathway Through PPAR-γ Activation in Cerulein-Induced Acute Pancreatitis and Associated Lung Injury in Mice. Inflammation 45, 45–58 (2022). https://doi.org/10.1007/s10753-021-01527-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01527-7