Abstract
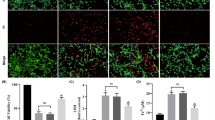
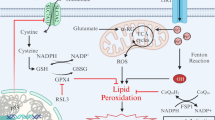
Host defenses in the brain are modulated by the activation of several factors such as oxygen free radical species (ROS), Ca2+ influx, and TRPM2 activation, and they are well-known adverse factors in neurotoxicity and neurodegenerative diseases. Importantly, recent data indicated a protective action of curcumin (CRC) via inhibition of TRPM2 on the inflammation factors, ROS, and apoptosis in hypoxia-induced SH-SY5Y neuronal cells. However, the relationship between interferon gamma (IFNg) exposure and TRPM2 activation in the SH-SY5Y cells are not fully identified. The SH-SY5Y cells as a neuronal cell line model were used in several neuroinflammation studies. Hence, we used the SH-SY5Y cells in the current study, and they were divided into four main groups as control, CRC, IFNg, and IFNg+CRC. The data presented here indicate that IFNg induced excessive Ca2+ influx via activation of TRPM2. The IFNg treatment further increased cell death, cell debris amount, apoptosis, and cytokine generations (IL-1β, IL-6, and TNF-α) which were due to increased cytosolic and mitochondrial ROS generations as well as increased activations of caspase-3 and caspase-9. The expression levels of TRPM2, PARP-1, Bax, caspase-3, and caspase-9 were increased in the cells by the IFNg treatment. However, CRC treatment reduced the increase of expression levels, cytokine generations, caspase activations, ROS release, Ca2+ influx, cell death, and apoptosis levels via inhibition of TRPM2 in the SH-SY5Y cells that were treated with IFNg. Moreover, the treatment of TRPM2 blockers (ACA and 2-APB) potentiated the modulator effects of CRC. In conclusion, these results suggest that neuroinflammation via IFNg lead to the TRPM2 activation in the SH-SY5Y cells, whereas CRC prevents IFNg-mediated TRPM2 activation, cell death, and cytokine generations.










Similar content being viewed by others
Data availability
The details of row data and materials are available on request from correspondence author (Prof. Dr. Mustafa Nazıroğlu). The graphics in the manuscript were prepared by the correspondence author.
References
Adami, C.R., R. Bianchi, G. Pula, and Donato R. 2004. S100B-stimulated NO production by BV-2 microglia is independent of RAGE transducing activity but dependent on RAGE extracellular domain. Biochim Biophys Acta 1742 (1-3): 169–177. https://doi.org/10.1016/j.bbamcr.2004.09.008.
Ronaldson, P.T., and . Davis T.P. 2020. Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J Cereb Blood Flow Metab 40 (1 suppl): S6–S24. https://doi.org/10.1177/0271678X20951995.
Yang, B., L.Y. Zhang, Y. Chen, Y.P. Bai, J. Jia, J.G. Feng, K.X. Liu, and J. Zhou. 2020. Melatonin alleviates intestinal injury, neuroinflammation and cognitive dysfunction caused by intestinal ischemia/reperfusion. Int Immunopharmacol 85: 106596. https://doi.org/10.1016/j.intimp.2020.106596.
Y., Akyuva, and Nazıroğlu M. 2020. Resveratrol attenuates hypoxia-induced neuronal cell death, inflammation and mitochondrial oxidative stress by modulation of TRPM2 channel. Sci Report 10 (1): 6449. https://doi.org/10.1038/s41598-020-63577-5.
Nazıroğlu, M., A. Öz, and K. Yıldızhan. 2020. Selenium and neurological diseases: Focus on peripheral pain and TRP channels. Curr Neuropharmacol 18 (6): 501–517. https://doi.org/10.2174/1570159X18666200106152631.
Rajasekar, N.S., S.K. Dwivedi, P.K. Kamat Tota, Hanif K., Nath C., and Shukla R. 2013. Neuroprotective effect of curcumin on okadaic acid induced memory impairment in mice. Eur J Pharmacol 715 (1-3): 381–394. https://doi.org/10.1016/j.ejphar.2013.04.033.
Roychowdhury, S., Wolf G., Keilhoff G., and Horn T.F. 2003. Cytosolic and mitochondrial glutathione in microglial cells are differentially affected by oxidative/nitrosative stress. Nitric Oxide 8 (1): 39–47. https://doi.org/10.1016/S1089-8603(02)00146-5.
Yıldızhan, K., and Nazıroğlu M. 2019. Microglia and its role in neurodegenerative diseases. Journal of Cellular Neuroscience Oxidative Stress 11: 861–873. https://doi.org/10.37212/jcnos.683407.
Egger, F., M. Jakab, J. Fuchs, K. Oberascher, G. Brachtl, M. Ritter, H.H. Kerschbaum, and M. Gaisberger. 2020. Effect of glycine on BV-2 microglial cells treated with interferon-γ and lipopolysaccharide. Int J Mol Sci 26 (21(3)): E804. https://doi.org/10.3390/ijms21030804.
Monteiro, S., S. Roque, F. Marques, M. Correia-Neves, and J.J. Cerqueira. 2017. Brain interference: Revisiting the role of IFNγ in the central nervous system. Prog Neurobiol 156: 149–163. https://doi.org/10.1016/j.pneurobio.2017.05.003.
Ebadi, M., and S.K. Sharma. 2003. Peroxynitrite and mitochondrial dysfunction in the pathogenesis of Parkinson’s disease. Antioxid Redox Signal 5 (3): 319–335. https://doi.org/10.1089/152308603322110896.
Titze-de-Almeida, S.S., C.F. Lustosa, C.H. Horst, E.D. Bel, and R. Titze-de-Almeida. 2014. Interferon gamma potentiates the injury caused by MPP(+) on SH-SY5Y cells, which is attenuated by the nitric oxide synthases inhibition. Neurochem Res 39 (12): 2452–2464. https://doi.org/10.1007/s11064-014-1449-1.
Du, Y., M. Fu, Y.T. Wang, and Z. Dong. 2018. Neuroprotective Effects of ginsenoside Rf on amyloid-β-Induced neurotoxicity in vitro and in vivo. Journal of Alzheimers Disease 64 (1): 309–322. https://doi.org/10.3233/JAD-180251.
Uddin, S.J., M.F. Hasan, M. Afroz, D.K. Sarker, R. Rouf, M.T. Islam, J.A. Shilpi, and M.S. Mubarak. 2020. Curcumin and its multi-target function against pain and inflammation: An update of pre-clinical data. Curr Drug Targets 21 (Sep 25). https://doi.org/10.2174/1389450121666200925150022. Epub ahead of print.
Chen, S., R. Corteling, L. Stevanato, and J. Sinden. 2012. Natural inhibitors of indoleamine 3,5-dioxygenase induced by interferon-gamma in human neural stem cells. Biochem Biophys Res Commun 429 (1-2): 117–123. https://doi.org/10.1016/j.bbrc.2012.10.009.
Liu, W., J. Yuan, H. Zhu, X. Zhang, L. Li, X. Liao, Z. Wen, Y. Chen, H. Feng, and J. Lin. 2016. Curcumin reduces brain-infiltrating T lymphocytes after intracerebral hemorrhage in mice. Neurosci Lett 620 (2016): 74–82. https://doi.org/10.1016/j.neulet.2016.03.047.
Keshk, W.A., W.S. Elseady, N.I. Sarhan, and D.H. Zineldeen. 2020. 2020. Curcumin attenuates cytoplasmic/endoplasmic reticulum stress, apoptosis and cholinergic dysfunction in diabetic rat hippocampus. Metab Brain Dis 35 (4): 637–647. https://doi.org/10.1007/s11011-020-00551-0.
Armağan, H.H., and M. Nazıroğlu. 2020. Curcumin attenuates hypoxia-induced oxidative neurotoxicity, apoptosis, calcium, and zinc ion influxes in a neuronal cell line: Involvement of TRPM2 channel. Neurotox Res. https://doi.org/10.1007/s12640-020-00314-w.
Nazıroğlu, M., and A. Demirdaş. 2015. Psychiatric disorders and TRP channels: Focus on psychotropic drugs. Curr Neuropharmacol 13 (2): 248–257. https://doi.org/10.2174/1570159x13666150304001606.
Nazıroğlu, M. 2007. New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32 (11): 1990–2001. https://doi.org/10.1007/s11064-007-9386-x.
Perraud, A.L., A. Fleig, C.A. Dunn, L.A. Bagley, P. Launay, C. Schmitz, A.J. Stokes, Q. Zhu, M.J. Bessman, R. Penner, J.P. Kinet, and A.M. Scharenberg. 2001. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411 (6837): 595–599. https://doi.org/10.1038/35079100.
Hara, Y., M. Wakamori, M. Ishii, E. Maeno, M. Nishida, T. Yoshida, H. Yamada, S. Shimizu, E. Mori, J. Kudoh, N. Shimizu, H. Kurose, Y. Okada, K. Imoto, and Y. Mori. 2002. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9 (1): 163–173. https://doi.org/10.1016/S1097-2765(01)00438-5.
Nazıroğlu, M., and A. Lückhoff. 2008. Effects of antioxidants on calcium influx through TRPM2 channels in transfected cells activated by hydrogen peroxide. J Neurol Sci 270 (1-2): 152–158. https://doi.org/10.1016/j.jns.2008.03.003.
Kraft, R., C. Grimm, H. Frenzel, and C. Harteneck. 2006. Inhibition of TRPM2 cation channels by N-(p-amylcinnamoyl)anthranilic acid. Br J Pharmacol 148 (3): 264–273.
Togashi, K., H. Inada, and M. Tominaga. 2008. Inhibition of the transient receptor potential cation channel TRPM2 by 2-aminoethoxydiphenyl borate (2-APB). Br J Pharmacol 153 (6): 1324–1330. https://doi.org/10.1038/sj.bjp.0707675.
Abuarab, N., T.S. Munsey, L.H. Jiang, J. Li, and A. Sivaprasadarao. 2017. High glucose-induced ROS activates TRPM2 to trigger lysosomal membrane permeabilization and Zn2+-mediated mitochondrial fission. Sci Signal 10 ((490)): eaal4161. https://doi.org/10.1126/scisignal.aal4161.
Osmanlıoğlu, H.Ö., M.K. Yıldırım, Y. Akyuva, K. Yıldızhan, and M. Nazıroğlu. 2020. Morphine induces apoptosis, inflammation, and mitochondrial oxidative stress via activation of TRPM2 channel and nitric oxide signaling pathways in the hippocampus. Mol Neurobiol 57 (8): 3376–3389. https://doi.org/10.1007/s12035-020-01992-5.
Zhu, T., Y. Zhao, H. Hu, Q. Zheng, X. Luo, Y. Ling, Y. Ying, Z. Shen, P. Jiang, and Q. Shu. 2019. TRPM2 channel regulates cytokines production in astrocytes and aggravates brain disorder during lipopolysaccharide-induced endotoxin sepsis. Int Immunopharmacol 75: 105836. https://doi.org/10.1016/j.intimp.2019.105836.
Akyuva, Y., M. Nazıroğlu, and K. Yıldızhan. 2021. Selenium prevents interferon-gamma induced activation of TRPM2 channel and inhibits inflammation, mitochondrial oxidative stress, and apoptosis in microglia. Metab Brain Dis 36 (2): 285–298. https://doi.org/10.1007/s11011-020-00624-0.
Yıldızhan, K., and M. Nazıroğlu. 2020. Glutathione depletion and parkinsonian neurotoxin MPP+-induced TRPM2 channel activation play central roles in oxidative cytotoxicity and inflammation in Microglia. Mol Neurobiol 57 (8): 3508–3525. https://doi.org/10.1007/s12035-020-01974-7.
Bao, L., S.J. Chen, K. Conrad, K. Keefer, T. Abraham, J.P. Lee, J. Wang, X.Q. Zhang, I. Hirschler-Laszkiewicz, H.G. Wang, S. Dovat, B. Gans, M. Madesh, J.Y. Cheung, and B.A. Miller. 2016. Depletion of the human ion channel TRPM2 in neuroblastoma demonstrates its key role in cell survival through modulation of mitochondrial reactive oxygen species and bioenergetics. J Biol Chem 291 (47): 24449–24464. https://doi.org/10.1074/jbc.M116.747147.
Uğuz, A.C., A. Öz, and M. Nazıroğlu. 2016. Curcumin inhibits apoptosis by regulating intracellular calcium release, reactive oxygen species and mitochondrial depolarization levels in SH-SY5Y neuronal cells. Journal of Receptors and Signal Transduction 36 (4): 395–401. https://doi.org/10.3109/10799893.2015.1108337.
Akpınar, O., A. Özşimşek, M. Güzel, and M. Nazıroğlu. 2020. Clostridium botulinum neurotoxin A induces apoptosis and mitochondrial oxidative stress via activation of TRPM2 channel signaling pathway in neuroblastoma and glioblastoma tumor cells. Journal of Receptors and Signal Transduction 40 (6): 620–632. https://doi.org/10.1080/10799893.2020.1781174.
Liu, Z.J., W. Zhao, H.Y. Lei, H.L. Xu, L.Y. Lai, R. Xu, and S.Y. Xu. 2019. High glucose enhances bupivacaine-induced neurotoxicity via MCU-mediated oxidative stress in SH-SY5Y Cells. Oxidative Med Cell Longev 2019: 7192798. https://doi.org/10.1155/2019/7192798,11.
Gökçe Kütük, S., G. Gökçe, M. Kütük, H.E. Gürses Cila, and M. Nazıroğlu. 2019. Curcumin enhances cisplatin-induced human laryngeal squamous cancer cell death through activation of TRPM2 channel and mitochondrial oxidative stress. Sci Rep 9 (1): 17784. https://doi.org/10.1038/s41598-019-54284-x.
Nazıroğlu, M., B. Çiğ, Y. Yazğan, G.K. Schwaerzer, F. Theilig, and L. Pecze. 2019. Albumin evokes Ca2+-induced cell oxidative stress and apoptosis through TRPM2 channel in renal collecting duct cells reduced by curcumin. Sci Rep 9 (1): 12403. https://doi.org/10.1038/s41598-019-48716-x.
Hashioka, S., A. Klegeris, C. Schwab, and P.L. McGeer. 2009. Interferon-gamma-dependent cytotoxic activation of human astrocytes and astrocytoma cells. Neurobiol Aging 30 (12): 1924–1935. https://doi.org/10.1016/j.neurobiolaging.2008.02.019.
McHugh, D., R. Flemming, S.Z. Xu, A.L. Perraud, and D.J. Beech. 2003. Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J Biol Chem 278 (13): 11002–11006.
Joshi, D.C., and J.C. Bakowska. 2011. Determination of mitochondrial membrane potential and reactive oxygen species in live rat cortical neurons. J Vis Exp 51: 2704. https://doi.org/10.3791/2704.
Ertilav, K. 2019. Pregabalin protected cisplatin-induced oxidative neurotoxicity in neuronal cell line. Journal of Cellular Neuroscience Oxidative Stress 11 ((1)): 815–824. https://doi.org/10.37212/jcnos.653500.
Özkaya, D., and M. Nazıroğlu. 2020. Curcumin diminishes cisplatin-induced apoptosis and mitochondrial oxidative stress through inhibition of TRPM2 channel signaling pathway in mouse optic nerve. Journal of Receptors and Signal Transduction 40 (2): 97–108. https://doi.org/10.1080/10799893.2020.1720240.
Miyake, T., H. Shirakawa, A. Kusano, S. Sakimoto, M. Konno, T. Nakagawa, Y. Mori, and S. Kaneko. 2014. TRPM2 contributes to LPS/IFNγ-induced production of nitric oxide via the p38/JNK pathway in microglia. Biochem Biophys Res Commun 444 (2): 212–217. https://doi.org/10.1016/j.bbrc.2014.01.022.
Kaur, N., B. Lu, R.K. Monroe, S.M. Ward, and S.W. Halvorsen. 2005. Inducers of oxidative stress block ciliary neurotrophic factor activation of Jak/STAT signaling in neurons. J Neurochem 92 (6): 1521–1530. https://doi.org/10.1111/j.1471-4159.2004.02990.x.
Engin, A.B., E.D. Engin, K. Golokhvast, D.A. Spandidos, and A.M. Tsatsakis. 2017. Glutamate-mediated effects of caffeine and interferon-γ on mercury-induced toxicity. Int J Mol Med 39 (5): 1215–1223. https://doi.org/10.3892/ijmm.2017.2937.
Aminzadeh, M., M. Roghani, A. Sarfallah, and G.H. Riazi. 2018. TRPM2 dependence of ROS-induced NLRP3 activation in Alzheimer’s disease. Int Immunopharmacol 54: 78–85. https://doi.org/10.1016/j.intimp.2017.10.024.
Wang, Z., W. Ren, F. Zhao, Y. Han, C. Liu, and K. Jia. 2020. Curcumin amends Ca2+ dysregulation in microglia by suppressing the activation of P2X7 receptor. Mol Cell Biochem 465 (1-2): 65–73. https://doi.org/10.1007/s11010-019-03668-8.
Ly, J.D., D.R. Grubb, and A. Lawen. 2003. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis 8 (2): 115–128. https://doi.org/10.1023/a:1022945107762.
Peng, Z., D.W. Luchtman, X. Wang, Y. Zhang, and C. Song. 2019. Activation of microglia synergistically enhances neurodegeneration caused by MPP+ in human SH-SY5Y cells. Eur J Pharmacol 850: 64–74. https://doi.org/10.1016/j.ejphar.2019.01.024.
Espino, J., I. Bejarano, P.C. Redondo, J.A. Rosado, C. Barriga, R.J. Reiter, J.A. Pariente, and A.B. Rodríguez. 2010. Melatonin reduces apoptosis induced by calcium signaling in human leukocytes: Evidence for the involvement of mitochondria and Bax activation. J Membr Biol 233 (1-3): 105–118. https://doi.org/10.1007/s00232-010-9230-0.
Öz, A., and Ö. Çelik. 2016. Curcumin inhibits oxidative stress-induced TRPM2 channel activation, calcium ion entry and apoptosis values in SH-SY5Y neuroblastoma cells: Involvement of transfection procedure. Mol Membr Biol 33 (3-5): 76–88. https://doi.org/10.1080/09687688.2017.1318224.
Kheradpezhouh, E., G.J. Barritt, and G.Y. Rychkov. 2016. Curcumin inhibits activation of TRPM2 channels in rat hepatocytes. Redox Biol 7: 1–7. https://doi.org/10.1016/j.redox.2015.11.001.
Kaur, D., V. Sharma, and R. Deshmukh. 2019. Activation of microglia and astrocytes: A roadway to neuroinflammation and Alzheimer's disease. Inflammopharmacology 27 (4): 663–677. https://doi.org/10.1007/s10787-019-00580-x.
Chiu, Y.J., Y.H. Hsieh, T.H. Lin, G.C. Lee, H.M. Hsieh-Li, Y.C. Sun, C.M. Chen, K.H. Chang, and G.J. Lee-Chen. 2019. Novel compound VB-037 inhibits Aβ aggregation and promotes neurite outgrowth through enhancement of HSP27 and reduction of P38 and JNK-mediated inflammation in cell models for Alzheimer’s disease. Neurochem Int 125: 175–186. https://doi.org/10.1016/j.neuint.2019.01.021.
Acknowledgements
The authors wish thanks to Prof. Dr. Peter Butterworth (King’s College, London, UK) for polishing English of the manuscript.
Funding
This study was carried out with financial support from BSN Health, Analyses, Innovation, Consultancy, Organization, Agriculture, Industry Ltd. (Göller Bölgesi Teknokenti, Isparta, Turkey). (Project No: 2019-06).
Author information
Authors and Affiliations
Contributions
The cell culture, spectrophotometer (antioxidant), and plate reader (cell viability, apoptosis, and caspase) analyses in the current study were performed in 3rd International Brain Research School, 25 June-1 July 2018, Isparta, Turkey, by Dr. Mustafa Güzel and Assoc. Prof. Dr. Orhan Akpınar. (http://2018.brs.org.tr//). Western blot analyses were performed by Ramazan Çınar (PhD Student of Department of Neuroscience). Laser confocal microscope analyses were performed by the correspondence author.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
There is no human or animal participant in the current study. The study was performed in the commercial cell line. The manuscript submission was approved by the authors.
Consent for Publication
Last version of the manuscript was approved by the authors before the submission.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Güzel, M., Nazıroğlu, M., Akpınar, O. et al. Interferon Gamma-Mediated Oxidative Stress Induces Apoptosis, Neuroinflammation, Zinc Ion Influx, and TRPM2 Channel Activation in Neuronal Cell Line: Modulator Role of Curcumin. Inflammation 44, 1878–1894 (2021). https://doi.org/10.1007/s10753-021-01465-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01465-4