Abstract
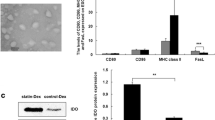
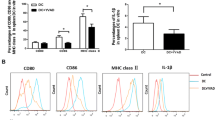
Myasthenia gravis (MG) is a neurological autoimmune disease characterized by fluctuating weakness of certain voluntary muscles. Current treatments for MG are largely directed at suppressing the whole immune system by using immunosuppressants or glucocorticoids and often cause several side effects. The ideal therapeutic methods for MG should suppress aberrant immunoactivation specifically, while retaining normal function of the immune system. In this study, we first produced exosomes from microRNA-146a overexpressing dendritic cells (DCs). Then, we observed suppressive effects of those exosomes in experimental autoimmune myasthenia gravis (EAMG) mice. Results showed that exosomes from microRNA-146a overexpressing DCs expressed decreased levels of CD80 and CD86. In experimental autoimmune MG, exosomes from microRNA-146a overexpressing DCs suppressed ongoing clinical MG in mice and altered T helper cell profiles from Th1/Th17 to Th2/Treg both in serum and spleen, and the therapeutic effects of those exosomes were antigen-specific and partly dose dependent. All the findings provide experimental basis for antigen-specific therapy of MG.









Similar content being viewed by others
References
Christadoss, P., J. Lindstrom, S. Munro, and N. Talal. 1985. Muscle acetylcholine receptor loss in murine experimental autoimmune myasthenia gravis: Correlated with cellular, humoral and clinical responses. Journal of Neuroimmunology 8: 29–44.
Drachman, D.B. 1994. Myasthenia gravis. The New England Journal of Medicine 330: 1797–1810.
Rodgaard, A., F.C. Nielsen, R. Djurup, F. Somnier, and S. Gammeltoft. 1987. Acetylcholine receptor antibody in myasthenia gravis: Predominance of IgG subclasses 1 and 3. J Clin. Exp Immunol 67: 82–88.
Christadoss, P., M. Poussin, and C. Deng. 2000. Animal models of myasthenia gravis. Clinical Immunology 94: 75–87.
Imai, T., S. Suzuki, E. Tsuda, Y. Nagane, H. Murai, M. Masuda, S. Konno, Y. Suzuki, S. Nakane, K. Fujihara, N. Suzuki, and K. Utsugisawa. 2015. Oral corticosteroid therapy and present disease status in myasthenia gravis. Muscle & Nerve 51: 692–696.
Luo, J., and J. Lindstrom. 2014. Antigen-specific immunotherapeutic vaccine for experimental autoimmune myasthenia gravis. Journal of Immunology 193: 5044–5055.
Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392: 245–252.
Osorio, F., C. Fuentes, M.N. López, F. Salazar-Onfray, and F.E. González. 2015. Role of dendritic cells in the induction of lymphocyte tolerance. Frontiers in Immunology 6: 535.
Chung, C.Y., D. Ysebaert, Z.N. Berneman, and N. Cools. 2013. Dendritic cells: Cellular mediators for immunological tolerance. Clinical & Developmental Immunology 2013: 972865.
O’Connell, R.M., D.S. Rao, A.A. Chaudhuri, and D. Baltimore. 2010. Physiological and pathological roles for microRNAs in the immune system. Nature Reviews. Immunology 10: 111–122.
Fukaya, T., and Y. Tomari. 2012. MicroRNAs mediate gene silencing via multiple different pathways in Drosophila. Molecular Cell 48: 825–836.
Du, J., J. Wang, G. Tan, Z. Cai, L. Zhang, B. Tang, and Z. Wang. 2012. Aberrant elevated microRNA-146a in dendritic cells (DC) induced by human pancreatic cancer cell line BxPC-3-conditioned medium inhibits DC maturation and activation. Medical Oncology 29: 2814–2823.
Karrich, J.J., L.C. Jachimowski, M. Libouban, A. Iyer, K. Brandwijk, E.W. Taanman-Kueter, M. Nagasawa, E.C. de Jong, C.H. Uittenbogaart, and B. Blom. 2013. MicroRNA-146a regulates survival and maturation of human plasmacytoid dendritic cells. Blood 122: 3001–3009.
Park, H., X. Huang, C. Lu, M.S. Cairo, and X. Zhou. 2015. MicroRNA-146a and microRNA-146b regulate human dendritic cell apoptosis and cytokine production by targeting TRAF6 and IRAK1 proteins. The Journal of Biological Chemistry 290: 2831–2841.
Chen, T., Z. Li, T. Jing, W. Zhu, J. Ge, X. Zheng, X. Pan, H. Yan, and J. Zhu. 2011. MicroRNA-146a regulates the maturation process and pro-inflammatory cytokine secretion by targeting CD40L in oxLDL-stimulated dendritic cells. FEBS Letters 585: 567–573.
Bodey, B., Jr B. Bodey, S.E. Siegel, and H.E. Kaiser. 2000. Failure of cancer vaccines: The significant limitations of this approach to immunotherapy. Anticancer Research 20: 2665–2676.
Segura, E., S. Amigorena, and C. Théry. 2005. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells, Molecules & Diseases 35: 89.
Yin, W., S. Ouyang, Y. Li, B. Xiao, and H. Yang. 2013. Immature dendritic cell-derived exosomes: A promise subcellular vaccine for autoimmunity. Inflammation 36: 232–240.
Pêche, H., K. Renaudin, G. Beriou, E. Merieau, S. Amigorena, and M.C. Cuturi. 2006. Induction of tolerance by exosome and short-term immunosuppression in a fully MHC-mismatched rat cardiac allograft model. Am Transplantat 6: 1541–1550.
Yang, X., S. Meng, H. Jiang, C. Zhu, and W. Wu. 2011. Exosomes derived from immature bone marrow dendritic cells induce tolerogenicity of intestinal transplantation in rats. The Journal of Surgical Research 171: 826–832.
Kim, S.H., N. Bianco, R. Menon, E.R. Lechman, W.J. Shufesky, A.E. Morelli, and P.D. Robbins. 2006. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Molecular Therapy 13: 289–300.
Kim, S.H., N.R. Bianco, W.J. Shufesky, A.E. Morelli, and P.D. Robbins. 2007. Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. Journal of Immunology 179: 2242–2249.
Kim, S.H., E.R. Lechman, N. Bianco, R. Menon, A. Keravala, J. Nash, Z. Mi, S.C. Watkins, A. Gambotto, and P.D. Robbins. 2005. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. Journal of Immunology 174: 6440–6448.
Bu, N., H.Q. Wu, G.L. Zhang, S.Q. Zhan, R. Zhang, Q.Y. Fan, Y.L. Li, Y.F. Zhai, and H.W. Ren. 2015. Immature dendritic cell exosomes suppress experimental autoimmune myasthenia gravis. Journal of Neuroimmunology 285: 71–75.
Li, X.L., H. Li, M. Zhang, H. Xu, L.T. Yue, X.X. Zhang, S. Wang, C.C. Wang, Y.B. Li, Y.C. Dou, and R.S. Duan. 2016. Exosomes derived from atorvastatin-modified bone marrow dendritic cells ameliorate experimental autoimmune myasthenia gravis by up-regulated levels of IDO/Treg and partly dependent on FasL/Fas pathway. Journal of Neuroinflammation 13: 8.
Min, W.P., R. Gorczynski, X.Y. Huang, M. Kushida, P. Kim, M. Obataki, J. Lei, R.M. Suri, and M.S. Cattral. 2000. Dendritic cells genetically engineered to express Fas ligand induce donor-specific hyporesponsiveness and prolong allograft survival. Journal of Immunology 164: 161–167.
Doffek, K., X. Chen, S.L. Sugg, and J. Shilyansky. 2011. Phosphatidylserine inhibits NF-κB and p38 MAPK activation in human monocyte derived dendritic cells. Molecular Immunology 48: 1771–1777.
Matsue H, Yang C, Matsue K, Edelbaum D, Mummert M, Takashima A Contrasting impacts of immunosuppressive agents (rapamycin, FK506, cyclosporin A, and dexamethasone) on bidirectional dendritic cell-T cell interaction during antigen presentation. J Immunol 169:3555–3564.
Manavella, P.A., and I. Rubio-Somoza. 2011. Engineering elements for gene silencing: The artificial microRNAs technology. Methods in Molecular Biology 732: 121–123.
Pusic, A.D., K.M. Pusic, B.L. Clayton, and R.P. Kraig. 2014. IFNγ-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. Journal of Neuroimmunology 266: 12–23.
Li, X., J.J. Li, J.Y. Yang, D.S. Wan, W. Zhao, W.J. Song, W.M. Li, J.F. Wang, W. Han, Z.C. Zhang, Y. Yu, D.Y. Cao, and K.F. Dou. 2012. Tolerance induction by exosomes from immature dendritic cells and rapamycin in a mouse cardiac allograft model. PloS One 7: e44045.
Hall, B.M. 2015. T cell: Soldiers and spies—The surveillance and control of effector T cells by regulatory T cell. Clinical Journal of the American Society of Nephrology 10 (11): 2050–2064.
Talaat, R.M., S.F. Mohamed, I.H. Bassyouni, and A.A. Raouf. 2015. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: Correlation with disease activity. Cytokine 72 (2): 146–153.
Xiao, J., F. Zhu, X. Liu, and J. Xiong. 2012. Th1/Th2/Th17/Treg expression in cultured PBMCs with antiphospholipid antibodies. Molecular Medicine Reports 6 (5): 1035–1039.
Alexandre, P.B., D.B. Rodrigues, and V. Rodrigues. 2015. Expression pattern of transcription factors and intracellular cytokines reveals that clinically cured tuberculosis is accompanied by an increase in Mycobacterium-specific Th1, Th2, and Th17 cells. BioMed Research International 2015: 591237.
Pitt, J.M., F. André, S. Amigorena, J.C. Soria, A. Eggermont, G. Kroemer, and L. Zitvogel. 2016. Dendritic cell-derived exosomes for cancer therapy. The Journal of Clinical Investigation 126 (4): 1224–1232.
Tan, A., H. De La Peña, and A.M. Seifalian. 2010. The application of exosomes as a nanoscale cancer vaccine. International Journal of Nanomedicine 10 (5): 889–900.
Acknowledgements
This work was supported by the National Nature Science Foundation of China (No. 81571173 and No. 81601048).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, W., Ouyang, S., Luo, Z. et al. Immature Exosomes Derived from MicroRNA-146a Overexpressing Dendritic Cells Act as Antigen-Specific Therapy for Myasthenia Gravis. Inflammation 40, 1460–1473 (2017). https://doi.org/10.1007/s10753-017-0589-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0589-2