Abstract
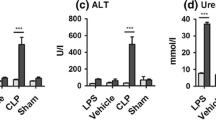
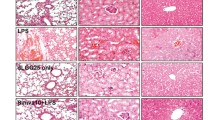
Plasma concentration of lysophosphatidylcholine (LPC) was reported to decrease in patients with sepsis. However, the mechanisms of sepsis-induced decrease in plasma LPC levels are not currently well known. In mice subjected to cecal ligation and puncture (CLP), a model of polymicrobial peritoneal sepsis, we examined alterations in LPC-related metabolic parameters in plasma, i.e., the plasma concentration of LPC-related substances (i.e., phosphatidylcholine (PC) and lysophosphatidic acid (LPA)), and activities or levels in the plasma of some enzymes that can be involved in the regulation of plasma LPC concentration (i.e., secretory phospholipase A2 (sPLA2), lecithin:cholesterol acyltransferase (LCAT), acyl-CoA:lysophosphatidylcholine acyltransferase (LPCAT), and autotaxin (ATX)), as well as plasma albumin concentration. We found that levels of LPC and albumin and enzyme activities of LCAT, ATX, and sPLA2 were decreased, whereas levels of PC, LPA, and LPCAT1–3 were increased in the plasma of mice subjected to CLP. Bacterial peritonitis led to alterations in all the measured LPC-related metabolic parameters in the plasma, which could potentially contribute to sepsis-induced decrease in plasma LPC levels. These findings could lead to the novel biomarkers of sepsis.





Similar content being viewed by others
References
Dial, E.J., D.M. Tran, J.J. Romero, M. Zayat, and L.M. Lichtenberger. 2010. A direct role for secretory phospholipase A2 and lysophosphatidylcholine in the mediation of LPS induced gastric injury. Shock 33(6): 634–638.
Schmid, B., M.J. Finnen, J.L. Harwood, and S.K. Jackson. 2003. Acylation of lysophosphatidylcholine plays a key role in the response of monocytes to lipopolysaccharide. European Journal of Biochemistry 270(13): 2782–2788.
Lin, P., E.J. Welch, X.-P. Gao, A.B. Malik, and R.D. Ye. 2005. Lysophosphatidylcholine modulates neutrophil oxidant production through elevation of cyclic AMP. Journal of Immunology 174(5): 2981–2989.
Yang, L.V., C.G. Radu, L. Wang, M. Riedinger, and O.N. Witte. 2005. Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood 105(3): 1127–1134.
Thies, F., M.C. Delachambre, M. Bentejac, M. Lagarde, and J. Lecerf. 1992. Unsaturated fatty acids esterified in 2‐acyl‐1‐lysophosphatidylcholine bound to albumin are more efficiently taken up by the young rat brain than the unesterified form. Journal of Neurochemistry 59(3): 1110–1116.
Subramanian, V.S., J. Goyal, M. Miwa, J. Sugatami, M. Akiyama, M. Liu, and P.V. Subbaiah. 1999. Role of lecithin-cholesterol acyltransferase in the metabolism of oxidized phospholipids in plasma: Studies with platelet-activating factor-acetyl hydrolase- deficient plasma. Biochimica et Biophysica Acta 1439(1): 95–109.
Taniyama, Y., S. Shibata, S. Kita, K. Horikoshi, H. Fuse, H. Shirafuji, Y. Sumino, and M. Fujino. 1999. Cloning and expression of a novel lysophospholipase which structurally resembles lecithin cholesterol acyltransferase. Biochemical and Biophysical Research Communications 257(1): 50–56.
Slotboom, A.J., and G.H. De Haas. 1970. Hydrolysis of phosphoglycerides by purified lipase preparations II. Preparation of unsaturated 2-monoacyl choline phosphoglycerides. Chemistry and Physics of Lipids 4(1): 30–36.
McKean, M.L., J.B. Smith, and M.J. Silver. 1981. Formation of lysophosphatidylcholine by human platelets in response to thrombin. Support for the phospholipase A2 pathway for the liberation of arachidonic acid. Journal of Biological Chemistry 256(4): 1522–1524.
Mehta, D., S. Gupta, S.N. Gaur, S.V. Gangal, and K.P. Agrawal. 1990. Increased leukocyte phospholipase A2 activity and plasma lysophosphatidylcholine levels in asthma and rhinitis and their relationship to airway sensitivity to histamine. The American Review of Respiratory Disease 142(1): 157–161.
Eder, A.M., T. Sasagawa, M. Mao, J. Aoki, and G.B. Mills. 2000. Constitutive and lysophosphatidic acid (LPA)-induced LPA production: Role of phospholipase D and phospholipase A2. Clinical Cancer Research 6(6): 2482–2491.
Lee, S., and K.R. Lynch. 2005. Brown recluse spider (Loxosceles reclusa) venom phospholipase D (PLD) generates lysophosphatidic acid (LPA). Biochemical Journal 391(2): 317.
Fourcade, O., M.F. Simon, C. Viodé, N. Rugani, F. Leballe, A. Ragab, B. Fournié, L. Sarda, and H. Chap. 1995. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell 80(6): 919–927.
Brown, W.J., K. Chambers, and A. Doody. 2003. Phospholipase A2 (PLA2) enzymes in membrane trafficking: Mediators of membrane shape and function. Traffic 4(4): 214–221.
Chen, X., B.A. Hyatt, M.L. Mucenski, R.J. Mason, and J.M. Shannon. 2006. Identification and characterization of a lysophosphatidylcholine acyltransferase in alveolar type II cells. Proceedings of the National Academy of Sciences 103(31): 11724–11729.
Nakanishi, H., H. Shindou, D. Hishikawa, T. Harayama, R. Ogasawara, A. Suwabe, R. Taguchi, and T. Shimizu. 2006. Cloning and characterization of mouse lung-type acyl-CoA:lysophosphatidylcholine acyltransferase 1 (LPCAT1). Expression in alveolar type II cells and possible involvement in surfactant production. The Journal of Biological Chemistry 281(29): 20140–20147.
Soupene, E., H. Fyrst, and F.A. Kuypers. 2008. Mammalian acyl-CoA:lysophosphatidylcholine acyltransferase enzymes. Proceedings of the National Academy of Sciences 105(1): 88–93.
Zhao, Y., Y.Q. Chen, T.M. Bonacci, D.S. Bredt, S. Li, W.R. Bensch, D.E. Moller, M. Kowala, R.J. Konrad, and G. Cao. 2008. Identification and characterization of a major liver lysophosphatidylcholine acyltransferase. Journal of Biological Chemistry 283(13): 8258–8265.
Bridges, J.P., M. Ikegami, L.L. Brilli, X. Chen, R.J. Mason, and J.M. Shannon. 2010. LPCAT1 regulates surfactant phospholipid synthesis and is required for transitioning to air breathing in mice. Journal of Clinical Investigation 120(5): 1736–1748.
Moessinger, C., L. Kuerschner, J. Spandl, A. Shevchenko, and C. Thiele. 2011. Human lysophosphatidylcholine acyltransferases 1 and 2 are located in lipid droplets where they catalyze the formation of phosphatidylcholine. The Journal of Biological Chemistry 286(24): 21330–21339.
Drobnik, W. 2003. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. Journal of Lipid Research 44(4): 754–761.
Cho, W.H., T. Park, Y.Y. Park, J.W. Huh, C.-M. Lim, Y. Koh, D.K. Song, and S.-B. Hong. 2011. Clinical significance of enzymatic lysophosphatidylcholine (LPC) assay data in patients with sepsis. European Journal of Clinical Microbiology and Infectious Diseases 31(8): 1805–1810.
Park, D.W., D.S. Kwak, Y.Y. Park, Y. Chang, J.W. Huh, C.M. Lim, Y. Koh, D.-K. Song, and S.-B. Hong. 2014. Impact of serial measurements of lysophosphatidylcholine on 28-day mortality prediction in patients admitted to the intensive care unit with severe sepsis or septic shock. Journal of Critical Care 29(5): 882.e885–882.e811.
Yan, J.-J., J.-S. Jung, J.-E. Lee, J. Lee, S.-O. Huh, H.-S. Kim, K.C. Jung, J.-Y. Cho, J.-S. Nam, H.-W. Suh, Y.-H. Kim, and D.K. Song. 2004. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nature Medicine 10(2): 161–167.
Bächner, D., M. Ahrens, N. Betat, D. Schröder, and G. Gross. 1999. Developmental expression analysis of murine autotaxin (ATX). Mechanisms of Development 84(1–2): 121–125.
Tokumura, A. 2002. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. The Journal of Biological Chemistry 277(42): 39436–39442.
Tokumura, A. 2004. Metabolic pathways and physiological and pathological significances of lysolipid phosphate mediators. Journal of Cellular Biochemistry 92(5): 869–881.
Kanda, H., R. Newton, R. Klein, Y. Morita, M.D. Gunn, and S.D. Rosen. 2008. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Natural Immunity 9(4): 415–423.
Moolenaar, W.H., and A. Perrakis. 2011. Insights into autotaxin: How to produce and present a lipid mediator. Nature Reviews Molecular Cell Biology 12(10): 674–679.
Fulkerson, Z., T. Wu, M. Sunkara, C.V. Kooi, A.J. Morris, and S.S. Smyth. 2011. Binding of autotaxin to integrins localizes lysophosphatidic acid production to platelets and mammalian cells. Journal of Biological Chemistry 286(40): 34654–34663.
Barlage, S., D. Fröhlich, A. Böttcher, M. Jauhiainen, H.P. Müller, F. Noetzel, G. Rothe, C. Schütt, R.P. Linke, K.J. Lackner, C. Ehnholm, and G. Schmitz. 2001. ApoE-containing high density lipoproteins and phospholipid transfer protein activity increase in patients with a systemic inflammatory response. Journal of Lipid Research 42(2): 281–290.
Shindou, H., D. Hishikawa, H. Nakanishi, T. Harayama, S. Ishii, R. Taguchi, and T. Shimizu. 2007. A single enzyme catalyzes both platelet-activating factor production and membrane biogenesis of inflammatory cells. Cloning and characterization of acetyl-CoA:LYSO-PAF acetyltransferase. The Journal of Biological Chemistry 282(9): 6532–6539.
Morishige, J., K. Touchika, T. Tanaka, K. Satouchi, K. Fukuzawa, and A. Tokumura. 2007. Production of bioactive lysophosphatidic acid by lysophospholipase D in hen egg white. Biochimica et Biophysica Acta 1771(4): 491–499.
Umezu-Goto, M. 2002. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. Journal of Cell Biology 158(2): 227–233.
van Meeteren, L.A. 2005. Inhibition of autotaxin by lysophosphatidic acid and sphingosine 1-phosphate. Journal of Biological Chemistry 280(22): 21155–21161.
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) (grant number 2015R1D1A1A01058532) and Hallym University Research Fund (grant number HRF-201606-011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The procedures for animal experiments were approved by the Animal Experimentation Committee at Hallym University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ahn, WG., Jung, JS., Kwon, H. et al. Alteration of Lysophosphatidylcholine-Related Metabolic Parameters in the Plasma of Mice with Experimental Sepsis. Inflammation 40, 537–545 (2017). https://doi.org/10.1007/s10753-016-0500-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0500-6