Abstract
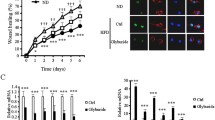
Diabetes frequently presents accumulation of advanced glycation end products (AGEs), which might induce excessive TNF-α production from macrophages to cause impaired wound healing. Recent studies have shown that activation of α7 nicotinic acetylcholine receptor (α7nAChR) on macrophages efficiently suppressed TNF-α synthesis. The aim of this study was to investigate the accumulation of AGEs in the wounds and determine whether PNU282987, an α7nAChR agonist, can improve wound repair by inhibiting AGE-mediated TNF-α production in a streptozotocin (STZ)-induced diabetic mouse model. Animals were assigned into four groups: wounded control group, wounded diabetic group, wounded diabetic group treated intraperitoneally with PNU282987, or wounded diabetic group treated intraperitoneally with vehicle. Compared with the non-diabetic control mice, the diabetic mice exhibited delayed wound healing that was characterized by elevated accumulation of AGEs, increased TNF-α level and macrophage infiltration, and decreased fibroblast number and collagen deposition at the late stage of repair. Besides, macrophages of diabetic wounds showed expression of α7nAChR. During late repair, PNU282987 treatment of diabetic mice significantly reduced the level of TNF-α, accelerated wound healing, and elevated fibroblast number and collagen deposition. To investigate the cellular mechanism of these observations, RAW 264.7 cells, a macrophage cell line, were incubated with AGEs in the presence or absence of PNU282987. TNF-α production from AGE-stimulated macrophages was significantly decreased by PNU282987 in a dose-dependent manner. Furthermore, PNU282987 significantly inhibited AGE-induced nuclear factor-κB (NF-κB) activation and receptor for AGE (RAGE) expression. These results strongly suggest that activating α7nAChR can promote diabetic wound healing by suppressing AGE-induced TNF-α production, which may be closely associated with the blockage of NF-κB activation in macrophages.











Similar content being viewed by others
References
Margolis, D.J., L. Allen-Taylor, O. Hoffstad, and J.A. Berlin. 2005. Diabetic neuropathic foot ulcers and amputation. Wound Repair and Regeneration 13: 230–236.
Jiang, Y., S. Huang, X. Fu, H. Liu, X. Ran, S. Lu, D. Hu, Q. Li, H. Zhang, Y. Li, R. Wang, T. Xie, B. Cheng, L. Wang, Y. Liu, X. Ye, C. Han, and H. Chen. 2011. Epidemiology of chronic cutaneous wounds in China. Wound Repair and Regeneration 19: 181–188.
Pierce, G.F. 2001. Inflammation in nonhealing diabetic wounds: the space–time continuum does matter. American Journal of Pathology 159: 399–403.
Wetzler, C., H. Kampfer, B. Stallmeyer, J. Pfeilschifter, and S. Frank. 2000. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse prolonged persistence of neutrophils and macrophages during the late phase of repair. Journal of Investigative Dermatology 115: 245–253.
Khanna, S., S. Biswas, Y. Shang, et al. 2010. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One 5, e9539.
Ebaid, H., O.M. Ahmed, A.M. Mahmoud, and R.R. Ahmed. 2013. Limiting prolonged inflammation during proliferation and remodeling phases of wound healing in streptozotocin-induced diabetic rats supplemented with camel undenatured whey protein. BMC Immunology 14: 31.
Desta, T., J. Li, T. Chino, and D.T. Graves. 2010. Altered fibroblast proliferation and apoptosis in diabetic gingival wounds. Journal of Dental Research 89: 609–614.
Siqueira, M.F., J. Li, L. Chehab, et al. 2010. Impaired wound healing in mouse models of diabetes is mediated by TNF-alpha dysregulation and associated with enhanced activation of forkhead box O1 (FOXO1). Diabetologia 53: 378–388.
Alikhani, M., Z. Alikhani, and D.T. Graves. 2005. FOXO1 functions as a master switch that regulates gene expression necessary for tumor necrosis factor-induced fibroblast apoptosis. Journal of Biological Chemistry 280: 12096–12102.
Xu, F., C. Zhang, and D.T. Graves. 2013. Abnormal cell responses and role of TNF-α in impaired diabetic wound healing. Biomed Research International 2013: 754802.
Goren, I., E. Muller, D. Schiefelbein, et al. 2007. Systemic anti-TNF alpha treatment restores diabetes-impaired skin repair in ob/ob mice by inactivation of macrophages. Journal of Investigative Dermatology 127: 2259–2267.
Sato, T., M. Iwaki, N. Shimogaito, X. Wu, S. Yamagishi, and M. Takeuchi. 2006. TAGE (toxic AGEs) theory in diabetic complications. Current Molecular Medicine 6: 351–358.
Takahashi, H.K., S. Mori, H. Wake, et al. 2009. Advanced glycation end products subspecies-selectively induce adhesion molecule expression and cytokine production in human peripheral blood mononuclear cells. Journal of Pharmacology and Experimental Therapeutics 330: 89–98.
Qin, Q., J. Niu, Z. Wang, et al. 2012. Astragalus membranaceus inhibits inflammation via phospho-P38 mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB pathways in advanced glycation end product-stimulated macrophages. International Journal of Molecular Sciences 13: 8379–8387.
Baek, G.H., Y.S. Jang, S.I. Jeong, J. Cha, M. Joo, S.W. Shin, K.T. Ha, and H.S. Jeong. 2012. Rehmannia glutinosa suppresses inflammatory responses elicited by advanced glycation end products. Inflammation 35: 1232–1241.
Choi, H.J., H.J. Jang, T.W. Chung, et al. 2013. Catalpol suppresses advanced glycation end-products-induced inflammatory responses through inhibition of reactive oxygen species in human monocytic THP-1 cells. Fitoterapia 86: 19–28.
Huijberts, M.S., N.C. Schaper, and C.G. Schalkwijk. 2008. Advanced glycation end products and diabetic foot disease. Diabetes/Metabolism Research and Reviews 24(Suppl 1): S19–24.
Roszer, T. 2011. Inflammation as death or life signal in diabetic fracture healing. Inflammation Research 60: 3–10.
Peppa, M., P. Stavroulakis, and S.A. Raptis. 2009. Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair and Regeneration 17: 461–472.
Goova, M.T., J. Li, T. Kislinger, et al. 2001. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. American Journal of Pathology 159: 513–525.
Wang, H., M. Yu, M. Ochani, C.A. Amella, M. Tanovic, S. Susarla, J.H. Li, H. Wang, H. Yang, L. Ulloa, Y. Al-Abed, C.J. Czura, and K.J. Tracey. 2003. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421: 384–388.
de Jonge, W.J., E.P. van der Zanden, The FO, M.F. Bijlsma, D.J. van Westerloo, R.J. Bennink, H.R. Berthoud, S. Uematsu, S. Akira, R.M. van den Wijngaard, and G.E. Boeckxstaens. 2005. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nature Immunology 6: 844–851.
Kurzen, H., I. Wessler, C.J. Kirkpatrick, K. Kawashima, and S.A. Grando. 2007. The non-neuronal cholinergic system of human skin. Hormone and Metabolic Research 39: 125–135.
Filippini, P., A. Cesario, M. Fini, F. Locatelli, and S. Rutella. 2012. The yin and yang of non-neuronal α7-nicotinic receptors in inflammation and autoimmunity. Current Drug Targets 13: 644–655.
Pavlov, V.A., M. Ochani, L.H. Yang, et al. 2007. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Critical Care Medicine 35: 1139–1144.
Marrero, M.B., M. Bencherif, P.M. Lippiello, et al. 2011. Application of alpha7 nicotinic acetylcholine receptor agonists in inflammatory diseases: an overview. Pharmaceutical Research 28: 413–416.
Fan, Y.Y., T.S. Yu, T. Wang, et al. 2011. Nicotinic acetylcholine receptor α7 subunit is time-dependently expressed in distinct cell types during skin wound healing in mice. Histochemistry and Cell Biology 135: 375–387.
Liu, L.P., T.H. Yan, L.Y. Jiang, W. Hu, M. Hu, C. Wang, Q. Zhang, Y. Long, J.Q. Wang, Y.Q. Li, M. Hu, and H. Hong. 2013. Pioglitazone ameliorates memory deficits in streptozotocin-induced diabetic mice by reducing brain β-amyloid through PPARγ activation. Acta Pharmacologica Sinica 34: 455–463.
Su, X., J.W. Lee, Z.A. Matthay, G. Mednick, T. Uchida, X. Fang, N. Gupta, and M.A. Matthay. 2007. Activation of the alpha7 nAChR reduces acid-induced acute lung injury in mice and rats. American Journal of Respiratory Cell and Molecular Biology 37: 186–192.
Waldburger, J.M., D.L. Boyle, V.A. Pavlov, K.J. Tracey, and G.S. Firestein. 2008. Acetylcholine regulation of synoviocyte cytokine expression by the alpha7 nicotinic receptor. Arthritis and Rheumatism 58: 3439–3449.
Shelukhina, I.V., E.V. Kryukova, K.S. Lips, V.I. Tsetlin, and W. Kummer. 2009. Presence of alpha7 nicotinic acetylcholine receptors on dorsal root ganglion neurons proved using knockout mice and selective alpha-neurotoxins in histochemistry. Journal of Neurochemistry 109: 1087–1095.
Shelukhina, I., R. Paddenberg, W. Kummer, and V. Tsetlin. 2015. Functional expression and axonal transport of α7 nAChRs by peptidergic nociceptors of rat dorsal root ganglion. Brain Structure and Function 220: 1885–1899.
Jones, I.W., and S. Wonnacott. 2004. Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. Journal of Neuroscience 24: 11244–11252.
Nemethova, A., K. Michel, P.J. Gomez-Pinilla, G.E. Boeckxstaens, and M. Schemann. 2013. Nicotine attenuates activation of tissue resident macrophages in the mouse stomach through the β2 nicotinic acetylcholine receptor. PLoS One 8, e79264.
Li, J., and A.M. Schmidt. 1997. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. Journal of Biological Chemistry 272: 16498–16506.
Jin, X., T. Yao, Z. Zhou, J. Zhu, S. Zhang, W. Hu, and C. Shen. 2015. Advanced glycation end products enhance macrophages polarization into M1 phenotype through activating RAGE/NF-κB pathway. Biomed Research International 2015: 732450.
Werner, S., and R. Grose. 2003. Regulation of wound healing by growth factors and cytokines. Physiological Reviews 83: 835–870.
Mahdavian Delavary, B., W.M. van der Veer, M. van Egmond, F.B. Niessen, and R.H. Beelen. 2011. Macrophages in skin injury and repair. Immunobiology 216: 753–762.
Buck, M., K. Houglum, and M. Chojkier. 1996. Tumor necrosis factor-alpha inhibits collagen alpha1(I) gene expression and wound healing in a murine model of cachexia. American Journal of Pathology 149: 195–204.
Ashcroft, G.S., M.J. Jeong, J.J. Ashworth, M. Hardman, W. Jin, N. Moutsopoulos, T. Wild, N. McCartney-Francis, D. Sim, G. McGrady, X.Y. Song, and S.M. Wahl. 2012. Tumor necrosis factor-alpha (TNF-α) is a therapeutic target for impaired cutaneous wound healing. Wound Repair and Regeneration 20: 38–49.
Singh, V.P., A. Bali, N. Singh, and A.S. Jaggi. 2014. Advanced glycation end products and diabetic complications. Korean Journal Physiology and Pharmacology 18: 1–14.
Zoheir, N., D.F. Lappin, and C.J. Nile. 2012. Acetylcholine and the alpha 7 nicotinic receptor: a potential therapeutic target for the treatment of periodontal disease? Inflammation Research 61: 915–926.
Herber, D.L., E.G. Severance, J. Cuevas, D. Morgan, and M.N. Gordon. 2004. Biochemical and histochemical evidence of nonspecific binding of alpha7nAChR antibodies to mouse brain tissue. Journal of Histochemistry and Cytochemistry 52: 1367–1376.
Moser, N., N. Mechawar, I. Jones, A. Gochberg-Sarver, A. Orr-Urtreger, M. Plomann, R. Salas, B. Molles, L. Marubio, U. Roth, U. Maskos, U. Winzer-Serhan, J.P. Bourgeois, A.M. Le Sourd, M. De Biasi, H. Schroder, J. Lindstrom, A. Maelicke, J.P. Changeux, and A. Wevers. 2007. Evaluating the suitability of nicotinic acetylcholine receptor antibodies for standard immunodetection procedures. Journal of Neurochemistry 102: 479–492.
Rommel, F.R., B. Raghavan, R. Paddenberg, W. Kummer, S. Tumala, G. Lochnit, U. Gieler, and E.M. Peters. 2015. Suitability of nicotinic acetylcholine receptor α7 and muscarinic acetylcholine receptor 3 antibodies for immune detection: evaluation in murine skin. Journal of Histochemistry and Cytochemistry 63: 329–339.
Schmidt, A.M., S.D. Yan, S.F. Yan, and D.M. Stern. 2001. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. Journal of Clinical Investigation 108: 949–955.
Xanthoulea, S., A. Deliaert, A. Romano, S.S. Rensen, W.A. Buurman, and R.R. van der Hulst. 2013. Nicotine effect on inflammatory and growth factor responses in murine cutaneous wound healing. International Immunopharmacology 17: 1155–1164.
Lee, R.H., and G. Vazquez. 2013. Evidence for a prosurvival role of alpha-7 nicotinic acetylcholine receptor in alternatively (M2)-activated macrophages. Physiological Reports 1, e00189.
Khan, M.A., M. Farkhondeh, J. Crombie, L. Jacobson, M. Kaneki, and J.A. Martyn. 2012. Lipopolysaccharide upregulates α7 acetylcholine receptors: stimulation with GTS-21 mitigates growth arrest of macrophages and improves survival in burned mice. Shock 38: 213–219.
Osborne-Hereford, A.V., S.W. Rogers, and L.C. Gahring. 2008. Neuronal nicotinic alpha7 receptors modulate inflammatory cytokine production in the skin following ultraviolet radiation. Journal of Neuroimmunology 193: 130–139.
Parrish, W.R., M. Rosas-Ballina, M. Gallowitsch-Puerta, M. Ochani, K. Ochani, L.H. Yang, L. Hudson, X. Lin, N. Patel, S.M. Johnson, S. Chavan, R.S. Goldstein, C.J. Czura, E.J. Miller, Y. Al-Abed, K.J. Tracey, and V.A. Pavlov. 2008. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Molecular Medicine 14: 567–574.
Sitapara, R.A., D.J. Antoine, L. Sharma, V.S. Patel, C.R. Ashby Jr., S. Gorasiya, H. Yang, M. Zur, and L.L. Mantell. 2014. The α7 nicotinic acetylcholine receptor agonist GTS-21 improves bacterial clearance in mice by restoring hyperoxia-compromised macrophage function. Molecular Medicine 20: 238–247.
Leite, P.E., J. Lagrota-Candido, L. Moraes, L. D’Elia, D.F. Pinheiro, R.F. da Silva, E.N. Yamasaki, and T. Quirico-Santos. 2010. Nicotinic acetylcholine receptor activation reduces skeletal muscle inflammation of mdx mice. Journal of Neuroimmunology 227: 44–51.
Leite, P.E., L. Gandía, R. de Pascual, C. Nanclares, I. Colmena, W.C. Santos, J. Lagrota-Candido, and T. Quirico-Santos. 2014. Selective activation of α7 nicotinic acetylcholine receptor (nAChRα7) inhibits muscular degeneration in mdx dystrophic mice. Brain Research 1573: 27–36.
Acknowledgments
This study was financially supported by grants from research funds for the Zhejiang Provincial Natural Science Foundation of China (LQ13H150002) and the National Natural Science Foundation of China (81301640).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Miao-Wu Dong and Ming Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Dong, MW., Li, M., Chen, J. et al. Activation of α7nAChR Promotes Diabetic Wound Healing by Suppressing AGE-Induced TNF-α Production. Inflammation 39, 687–699 (2016). https://doi.org/10.1007/s10753-015-0295-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0295-x