Abstract
Brachionus calyciflorus is a species complex consisting of four recently described species. Although several lines of evidence support their species status, hybridization between two of the sibling species B. calyciflorus s.s. and B. elevatus has been inferred from both field and laboratory studies. In this study, we tested for the existence of prezygotic barriers between these species by performing two types of cross-fertilization experiments. In a ‘mate competition’ experiment we exposed mictic females to equal numbers of conspecific and allospecific males and demonstrate that intraspecific fertilizations occur at much higher frequencies than interspecific fertilizations, providing evidence for a strong prezygotic reproductive barrier. This result was consistent across numerous combinations of parental genotypes. In addition, by creating interspecific combinations of mictic females and males in a ‘no choice’ experiment, we found that interspecific fertilization success is independent of fertilization direction while it does seem to depend on maternal genotype. Our results demonstrate the existence of a strong prezygotic barrier that may play an important role in the maintenance of species boundaries. Yet, the observation of hybrids also shows a potential for gene flow between the species through hybridization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ‘species’ is a fundamental concept in taxonomy, ecology and evolution. Although there exist many definitions of this concept (de Queiroz, 2007; Leliaert et al., 2014), the biological species concept (Dobzhansky, 1950; Mayr, 1942) is undoubtedly the most influential one. Mayr, (1942) defined the biological species as a group of interbreeding natural populations that are reproductively isolated from other such groups. Mayr, (1942) thus recognized species as a reproductively cohesive assemblage of populations emphasizing genetic relationships rather than morphological differences. To maintain the integrity of species boundaries, the development of reproductive isolation is a critical step in the speciation process.
Reproductive barriers are broadly classified as prezygotic and postzygotic, depending on whether they function before or after zygotes form. Prezygotic barriers include physiological or systemic barriers that prevent mating and fertilization (e.g. temporal, habitat, behavioral, mechanical and gametic isolation). Postzygotic barriers are manifested as zygote mortality, hybrid inviability and hybrid sterility. Reproductive isolation is usually the result of a combination of pre- and postzygotic barriers (Carrió & Güemes, 2014; Ostevik et al., 2016). Many studies suggest that prezygotic barriers contribute more to reducing interspecific gene flow than postzygotic barriers (Chin et al., 2019; Lackey & Boughman, 2017; Sánchez-Guillén et al., 2012). This is because prezygotic barriers are generally found to evolve more quickly (Sánchez-Guillén et al., 2014; Stelkens et al., 2010). Prezygotic barriers can effectively prevent waste of resources on unfavorable genomic combinations and thereby promote reproductive isolation of the parental species (Hopkins, 2013; Matute 2010). Conversely, in some species, postzygotic barriers seem to evolve at similar rates as prezygotic barriers (Coyne & Orr, 1997; Moyle et al., 2004) or even faster (Edmands et al., 2009; Jewell et al., 2012). In a recent review, Coughlan & Matute, (2020) discussed the relative importance of postzygotic barriers in the process of speciation. Elucidating how reproductive isolation is formed through different barriers is therefore important for understanding the dynamics of speciation.
Many closely related species are, nevertheless, known to show porous species boundaries. Interspecific hybridization is increasingly recognized to be a very widespread phenomenon in plants (Gross & Rieseberg, 2005; Whitney et al., 2010), animals (Lamichhaney et al., 2018; Mavárez & Linares 2008) and fungi (Giraud et al. 2008; Staats et al., 2004). Up to 25% of vascular plant species hybridize with at least one other species in the wild (Mallet, 2005). In animals, predictions of interspecies hybridization frequencies range from approximately 1% (0.1–3%) (Schwenk et al., 2008) to 10% (Mallet, 2005). Hybridization is a very important phenomenon that may slow down or even reverse speciation via the homogenizing effect of gene flow (Behm et al., 2010; Gilman & Behm, 2011; Seehausen et al., 2008). Conversely, it may also accelerate speciation through adaptive introgression (Nichols et al., 2015; Rheindt et al., 2014), transgressive segregation (Holzman & Hulsey, 2017; Kagawa & Takimoto, 2018), or even cause near-instantaneous speciation by allopolyploidization (Vallejo-Marín et al., 2015; Wu et al., 2016).
Rotifera is an old phylum that can be traced back to at least 100 million years ago (Mark Welch et al., 2008). Although more than 2000 species have so far been described (Serra et al. 2019) this number is rapidly increasing due to the continuous discovery of new cryptic species complexes (Fontaneto, 2014; Kimpel et al., 2015; Kordbacheh et al., 2019; Moreno et al., 2017; Obertegger et al., 2014). Monogononta, accounting for more than three-quarters of rotifer species, is a group of cyclic parthenogenetic rotifers predominantly found in freshwater but also in soil and marine environments. In the ‘amictic’ or asexual stage, females produce diploid, subitaneous eggs that directly develop into genetically identical female offspring. Following certain stimuli (e.g., crowding, change of photoperiod or temperature), chemical signals are released to initiate the ‘mictic’ or sexual stage (Snell et al., 2006). Mictic females are morphologically similar to amictic females but produce haploid eggs via meiosis. If young mictic females are fertilized, they will produce dormant propagules, otherwise the haploid eggs will develop into males. Dormant propagules are very resistant to harsh environmental conditions, including cold and desiccation. When conditions turn favorable, a fraction of the dormant propagules will hatch and give rise to amictic females. Unhatched dormant propagules accumulate in the sediment, forming dormant propagule banks that may remain viable for a long time (Piscia et al., 2012).
Brachionus calyciflorus Pallas, 1766, a monogonont rotifer, was first suggested to be a species complex by Gilbert & Walsh, (2005). Since then, plenty of studies have addressed evolutionary divergence among different genetic lineages, studying aspects of molecular phylogeny (García-Morales & Elías-Gutiérrez, 2013; Papakostas et al., 2016), ecology (Paraskevopoulou et al., 2018; Zhang et al., 2019 and Zhang et al., 2018), phylogeography (Xiang et al., 2011a, 2011b), and reproductive isolation (Li et al., 2008; Xiang et al., 2011a, 2011b). Based on a comprehensive analysis of all publicly available sequence data of the molecular markers COI and ITS1, Papakostas et al., (2016) suggested the existence of four species, which were later confirmed via a morphometric analysis by Michaloudi et al., (2018) and formally (re-)described as B. calyciflorus s.s. Pallas, 1766, B. dorcas Gosse, 1851, B. elevatus Michaloudi, Papakostas, Stamou, Neděla, Tihlaříková, Zhang & Declerck, 2018 and B. fernandoi Michaloudi, Papakostas, Stamou, Neděla, Tihlaříková, Zhang & Declerck, 2018. Molecular field surveys (Li et al., 2008; Papakostas et al., 2016; Zhang et al., 2018), however, revealed the widespread occurrence of mitonuclear discordance between different species pairs. Simulations suggested most of these discordances stem from hybridization and introgression rather than incomplete lineage sorting (Papakostas et al., 2016). Strong additional support for hybridization between the most closely related sibling species B. elevatus and B. calyciflorus s.s. was provided by recent laboratory experiments (Papakostas et al., 2016; Zhang & Declerck, 2022).
The occurrence of hybridization raises the question of how species boundaries are maintained in the face of hybridization and whether reproductive barriers exist. Zhang & Declerck, (2022) studied postzygotic barriers between B. elevatus and B. calyciflorus s.s. and found that hybrid dormant propagules suffer higher mortality than propagules produced by conspecific fertilization. With the current study, we aim to test the hypothesis that reproduction between B. calyciflorus s.s. and B. elevatus is also hampered by prezygotic reproductive barriers. For this, we performed two types of cross-fertilization experiments. The first type of experiment (‘mate competition’ experiment) consisted of a series of assays designed to test if prezygotic barriers arise from differences in the relative ability of allospecific males to fertilize mictic females compared to conspecific males. The second type of experiment (‘no choice’ mating experiment) aimed at studying to what extent interspecific fertilization success is determined by fertilization direction and parental genotype identity.
Materials and methods
Sample collection, clone establishment and stock culture maintenance
In this study, both species were represented by multiple clonal genotypes originating from different populations in The Netherlands. Surface sediments were collected from five permanent freshwater ponds during April 2016 (Table 1). Dormant propagules of B. calyciflorus species complex were isolated by applying the sugar flotation method described by Gómez & Carvalho, (2000). Dormant propagules were hatched under continuous light in petri dishes with demineralized water at room temperature (23 ± 1 °C). Dishes were checked at 12 h intervals and hatched females were used to establish clonal lines. They were transferred individually to wells of 24-well plates filled with 1 mL of chemostat cultured Chlamydomonas reinhardtii P.A.Dangeard, 1888, nom. cons. (1000 μmol C L−1) resuspended in WC medium (Guillard, 1975). After population sizes had grown larger than 5 individuals, they were transferred to 20 mL plastic cups with 8 mL food and further maintained as stock cultures at room temperature (23 ± 1 °C) under continuous light. Every other day, ten to fifteen females carrying parthenogenetic eggs were transferred to new cups with fresh food.
DNA extraction, ITS1 PCR, RFLPs and microsatellite genotyping
The species identity of stock cultures was identified using restriction fragment length polymorphisms (RFLPs) together with microsatellite genotyping. First, DNA was extracted from a single rotifer using the HotSHOT method described by Montero‐Pau et al. (2008). ITS1, between 296 and 313 bp, were amplified using the primers III: 5’-CACACCGCCCGTCGCTACTACCGATTG-3’ and VIII: 5’-GTGCGTTCGAAGTGTCGATGATCAA-3’ (Hwang et al., 2013). For RFLPs analysis, 3 μL of ITS1 PCR products were digested by 1 μL AluI and DraI (New England Biolabs, Inc.) separately and incubated overnight at 37 °C. B. elevatus and B. calyciflorus s.s. were identified by examining the pattern of DNA bands produced on 1% agarose gel as described by Papakostas et al., (2016).
Microsatellite analysis was applied to confirm the species identity of stock cultures and to assess the hybrid status of dormant propagules. Twelve microsatellite loci were amplified using the primers described by Declerck et al., (2015). Alleles at loci A9 and A15 are known to be species-specific (Papakostas et al., 2016) and were therefore used for species identification and the detection of F1 hybrids. Microsatellite analysis was conducted with an ABI Prism 3130 DNA Analyzer (Applied Biosystems, CA) and genotyping was done with GeneMapper v.5.0 software (Applied Biosystems, CA).
Experiments: general
All experiments were conducted in a food suspension of 1000 μmol C L−1 food (C. reinhardtii) at room temperature (23 ± 1 °C) and under continuous light. Different sets of B. calyciflorus s.s. and B. elevatus clones were used in each of the experiments (Supplementary Table S1).
Preparation of animals for experiments
For the clones used in an experiment, males and mictic females were induced by creating high-density cultures (Gilbert, 2007). Starting from animals from the stock cultures, populations were first grown in 500 mL flasks with abundant food (1000 μmol C L−1 C. reinhardtii) at 24 °C and under continuous light. Every other day the food was refreshed by decanting approximately 80% of the culture and filtering it over a mesh (90 µm in case of high culture densities, 60 µm in case of low culture densities). Subsequently, animals were washed from the mesh and suspended in fresh food suspension. For the ‘no choice’ and mate competition experiments, females carrying male or parthenogenetic eggs were isolated individually into 24-well plates with food and checked hourly. Their male and female offspring were individually transferred to new wells and used for fertilization assays within 4 h after their birth (Snell & Childress, 1987).
Mate competition experiment
For the mate competition experiment (Fig. 1a), we performed a total of 243 assays. In each assay, one female was offered ten males of which five males belonged to a different clone from the same species (to exclude potential inbreeding) and the other five males represented one clone of the other species. The males were first pooled in 0.5 mL of food suspension before the female was introduced. Subsequently, the females were visually checked at 12 h time intervals and classified into three types according to the eggs they produced: (a) amictic females producing parthenogenetic eggs, (b) unfertilized mictic females producing male eggs, or (c) fertilized mictic females producing dormant propagules. Females proved amictic in 146 of the assays. The remaining 97 assays with mictic females all represented unique clone combinations (Supplementary Table S2) and involved both directions with B. elevatus and B. calyciflorus s.s. providing the mictic female in 55 and 42 of the cases, respectively. Fertilized mictic females were further kept in culture by refreshing their food suspension every 24 h until they died. The dormant propagules produced throughout their life were counted, classified as normal or abnormal according to Gilbert, (2010) (Supplementary Figure S1) and genotyped with microsatellite analysis to assess whether they had been formed by interspecific or conspecific fertilization.

modified from Michaloudi et al. (2018) and de Beauchamp (1965), respectively. Black and white color represent different species. Males were not drawn to scale
Basic design of the cross-fertilization experiments. In the ‘mate competition experiment’ (a), a female was offered ten males of which five males belonged to a different clone from the same species while the other five males represented one clone of the other species. In the ‘no choice’ mating experiment (b), a female was mixed with five males from a same clone of the other species. For more details on the specific combinations of genotypes, we refer to Supplementary Table S2 and Table S4. The silhouettes represent the body shape of females and males
‘no choice’ mating experiment
For the ‘no choice’ mating experiment (Fig. 1b), we performed a total of 318 assays. In each assay, we confronted single females of one of the species with five males from a same clone from the other species. Using five clones for each species (Supplementary Table S1), this was done multiple times for all 25 pairwise clone combinations and in both fertilization directions (i.e. both B. calyciflorus s.s. as well as B. elevatus served as mothers in each clone combination; Supplementary Table S4). Females were combined with males within wells of 24-well plates with 0.5 mL of food suspension. Females proved mictic in 145 of the 318 cases and we managed to perform three assays with mictic females for 47 out of the 50 planned parental clone combinations (Supplementary Table S4). Fertilized females were monitored throughout their life span, and their dormant propagules were collected for assessing their viability (Gilbert, 2010) and hybrid status.
Statistics
To test for the existence of a prezygotic barrier, we used the data of the mate competition experiment and evaluated if intraspecific fertilization is more prone to occur than interspecific fertilization as judged from the hybrid status of the dormant propagules. For this, we applied a Fisher exact test comparing the odds ratio of interspecific to intraspecific fertilizations with a 1:1 expectation. Using the data of the ‘no choice’ experiment, we applied binomial logistic regression to test the effect of maternal genotype, paternal genotype and fertilization direction on the observed occurrences of successful and unsuccessful fertilizations (i.e. dormant propagules versus unfertilized sexual eggs). For this, we constructed generalized linear models using a logit link function and assuming a binomial distribution. The importance of the studied factors was evaluated by comparing the models with an intercept model using the Akaike Information Criterion (AIC) and the loglikelihood ratio test. Statistical analyses were performed in the R software environment 3.6.3 (R core team 2020). Generalized linear models were performed using the lme4 package (Bates et al. 2014).
Results
Mate competition experiment
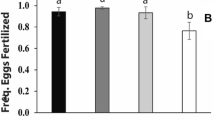
Of the 55 mictic B. elevatus females, eleven (20%) were successfully fertilized, judging from the production of dormant propagules (Supplementary Table S3). In B. calyciflorus s.s., fourteen (33%) of the 42 mictic females were fertilized. All the other females remained unfertilized given that they produced males. The fertilized females produced a total of 83 dormant propagules. Microsatellite analysis allowed assessment of the hybrid status of 70 of these dormant propagules (Supplementary Table S3) including at least one propagule of each of the fertilized females. There was only one case of interspecific hybridization, where a B. elevatus female (clone 101_IIIB) produced two hybrid dormant propagules (one normal and one abnormal) after being fertilized by a B. calyciflorus s.s. male (clone 128_IIC) (Supplementary Table S3). Hence, with 1/24, the ratio of hybrid to intraspecific fertilizations was substantially lower than a 1:1 ratio, providing compelling evidence for the existence of a strong prezygotic barrier in both fertilization directions (P < 0.001).
‘no choice’ mating experiment
Fertilization success tended to be determined by maternal genotype (χ2(9) = 21.7, P < 0.01) but not paternal genotype (Table 2). We found no evidence for an effect of fertilization direction. Three out of 72 of the mictic B. elevatus females (4%) were fertilized, two of which belonged to the same mother clone but mated with different father clones (Supplementary Table S4). From the 73 mictic B. calyciflorus s.s. females, four were fertilized (6%) and all belonged to the same mother clone (7_IIIC). Similar to B. elevatus, they were all fertilized by different father clones. Once fertilized, females produced multiple dormant propagules throughout the rest of their life span (Supplementary Table S4). The fractions of abnormal dormant propagules produced by B. elevatus and B. calyciflorus s.s. females were 75% and 62%, respectively. Microsatellite analysis on diagnostic loci A9 and A15 was successfully applied for 19 and 14 of the 25 dormant propagules, respectively and confirmed that all were hybrids (Supplementary Table S5).
Discussion
Our experiments demonstrate strong yet penetrable prezygotic barriers between B. calyciflorus s.s. and B. elevatus, two species of the B. calyciflorus species complex. On one hand, these findings reconfirm that B. calyciflorus s.s. and B. elevatus are at an advanced stage in the speciation process, which is well in line with the results of earlier phylogenetic, morphological, life history and ecological studies (Michaloudi et al., 2018; Papakostas et al., 2016; Paraskevopoulou et al., 2020, 2018; Zhang et al., 2019). On the other hand, they support earlier conclusions that these species do hybridize (Lemmen et al., 2020; Papakostas et al., 2016) providing a plausible explanation for frequently observed mitonuclear discordances in field populations (Papakostas et al., 2016; Zhang et al., 2019).
The mate competition experiment shows that intraspecific fertilizations are more likely to occur than interspecific fertilizations. Indeed, exposure of mictic females to equal numbers of conspecific and allospecific males resulted in a very strong bias towards intraspecific fertilization, given that on a total of 25 fertilized females only one female proved to produce hybrid eggs. Prezygotic barriers between species may originate from an inability to recognize each other’s sex signals. Monogonont rotifers are known for their peculiar species-specific mate recognition systems (Gómez & Snell, 1996; Snell et al., 1995). Mate recognition glycoprotein is located on the body surface of mictic females and males detect the signal by contact chemoreception through a corresponding receptor in their corona (Snell et al., 1995). Males search actively for mictic females but may fail to recognize them if they belong to a different species or select against them (Gómez and Snell, 1996; Snell & Stelzer, 2005). Furthermore, females may actively reject males that they don’t recognize as belonging to their own species (Gilbert & Walsh, 2005; Snell et al., 2007). The fact that interspecific fertilization does occur between B. calyciflorus s.s. and B. elevatus, nevertheless, indicates that there is still some level of recognition between species as well as tolerance towards deviations from the own species-specific signal. Alternatively, females are not always successful in fending off copulation with an allospecific male. Also, we cannot exclude the possibility that reduced interspecific fertilization success could also be related to sperm competition or genetic incompatibilities that result in premature abortion although we are not aware of any evidence for such mechanisms in rotifers yet. There also exist other types of prezygotic barriers that were out of the scope of the current study. Niche segregation in time or space (Schröder & Walsh, 2007; Zhang et al., 2018) may potentially contribute to the reproductive isolation of natural populations. Species may also be unable to recognize each other’s mixis inducing signal, i.e. the signal that induces parthenogenetically reproducing females to start producing mictic progeny (Gilbert, 2003; Snell, 2017; Stelzer & Snell, 2003). Although the latter form of reproductive isolation could also be of great importance in natural populations, its importance was not addressed in our study, given that all successful cross-fertilization experiments were already initiated with mictic females.
Our results also suggest that interspecific fertilization success has an important genetic component as we observed differences in interspecific fertilization frequencies between maternal genotypes. An important result of such genotype dependency may be a reduced genetic diversity of hybrid populations. In a field survey of hybridizing Daphnia galeata G.O. Sars, 1864 and Daphnia hyalina Leydig, 1860 populations, another cyclic parthenogenetic zooplankton taxon, Keller et al., (2007) detected significantly lower hybrid genotype diversity in ephippia (i.e. dormant propagules produced by Daphnia) than what would be expected from random mating. Along similar lines, in a field study of hybridizing populations of D. galeata and Daphnia longispina (O.F. Müller, 1776), Yin et al. (2012) found that clonal diversity in hybrid populations was significantly lower than in the populations of the parental species. Although yet to be verified empirically for the B. calyciflorus species complex, such reduced genetic diversity could potentially reduce the evolutionary potential of hybrid populations in the face of environmental change.
We found no evidence for asymmetrical hybridization. The ‘no choice’ mating experiments yielded hybrid eggs in similar numbers irrespective of whether mictic females were B. calyciflorus s.s. or B. elevatus. This contrasts with what has been found in, for example, the Daphnia pulex Leydig, 1860 × Daphnia pulicaria Forbes, 1893 species complex where the prezygotic barrier was found to be stronger in one than in the opposite direction (Chin et al., 2019). Similarly, asymmetrical hybridization was also found in the limited number of cases of successful hybridization between rotifers (Ruttner-Kolisko, 1969; Schröder & Walsh, 2007). For example, in the Epiphanes senta species complex, Epiphanes ukera Schröder & Walsh, 2007 males never fertilized females from the Epiphanes chihuahuaensis Schröder & Walsh, 2007 population whereas reciprocal hybridization was observed although mortality of juveniles was very high and those few that developed to adult females did not produce viable dormant propagules (Schröder & Walsh, 2007).
A number of studies have experimentally addressed prezygotic isolation between phylogenetically divergent lineages within the B. calyciflorus species complex (Gilbert & Walsh, 2005; Li et al., 2008; Xiang et al., 2011b). Although each of these studies reported evidence for reproductive isolation between phylogenetically divergent lineages, supporting the idea of cryptic species, they found no evidence for hybridization between such species. Due to issues related to the availability or quality of gene sequences, only for the Xiang et al., (2011b) study, we were able to unambiguously identify the identity of the investigated lineages according to the species (re-)described by Michaloudi et al., (2018). Gilbert and Walsh, (2005) evaluated reproductive isolation by studying the occurrence of male copulation behavior between four different strains. They observed copulation attempts only between strains with high degrees of similarity in ITS and COI sequences (0.3% sequence divergence), but never between more divergent lineages (7% and 9% sequence divergence, respectively). Li et al., (2008) and Xiang et al., (2011b) experimentally made interspecific combinations of males with mictic females and considered the production of dormant propagules as an indication for successful fertilization. Li et al., (2008) used five clones that belonged to three ITS1 lineages and found fertilization took place within most ITS1 lineages. Xiang et al., (2011b) cross-mated two B. calyciflorus s.s. and three B. fernandoi clones and only reported successful fertilization for intraspecific clone combinations. The latter results provide additional support for the idea that the species described by Michaloudi et al., (2018) are true species. However, the absence of any interspecific mating or fertilization is at odds with our observations of interspecific hybrid formation. Possibly, the number of clones involved and the number of trials performed in the other studies were too low to be able to detect such interspecific fertilization. Alternatively, there exist indications that B. calyciflorus s.s. and B. fernandoi are phylogenetically more distantly related than B. calyciflorus s.s. and B. elevatus (Papakostas et al., 2016) which may potentially result in stronger prezygotic barriers.
Despite the existence of important prezygotic (this study) and postzygotic barriers (Zhang and Declerck, 2022), there is convincing evidence for the occurrence of hybridization between B. calyciflorus s.s. and B. elevatus in field populations. A microsatellite analysis by Papakostas et al., (2016) revealed small fractions of F1 hybrids and possible backcrosses in field populations, whereas some hybrid clones have also been found to hatch from dormant propagules isolated from pond sediments (Zhang et al., 2019). This leads to the question which are the field conditions that may facilitate the formation of hybrids? We believe that our experimental results may provide some clues. Of the 145 mictic females that were confronted with males of the other species in the ‘no choice’ experiment, seven (i.e. 4.8%) produced hybrid dormant propagules. In contrast, of the 97 mictic females that were simultaneously exposed to males from both species in the mate competition experiment, only one (i.e. 1.0%) produced hybrid dormant propagules. This comparison seems to imply that the relative rates at which interspecific fertilizations happen depend on the relative abundance of allospecific to conspecific males. Admittedly, the numbers of successful fertilizations are low and it is also difficult to directly compare the outcomes of both results because they were obtained in two different experiments that made use of different combinations of clones. Nevertheless, these results may indicate that the probability of fertilization of a mictic female by a male from another species increases with an increasing allospecific to conspecific male abundance ratio. If this is true, we expect that the relative rate at which hybrid clones are created in natural populations of coexisting species will critically depend on: (1) the degree to which mixis induction in the species occurs simultaneously and (2) the relative abundance of the species when such simultaneous mixis happens. In cases where mixis of both species occur simultaneously while species differ strongly in relative abundances, relative rates of interspecific fertilizations may be high because mictic females from the least abundant species will be exposed to a higher abundance of allospecific compared to conspecific males. The latter scenario is especially likely if the most abundant species is able to induce mixis in the least abundant species. Unfortunately, we currently lack information on the phenology of mixis induction of the different species in natural populations and the extent to which they are able to induce mixis across species boundaries.
In conclusion, our results add to the morphological, ecological and phylogenetic evidence that B. calyciflorus s.s. and B. elevatus are different species. Strong prezygotic barriers effectively prevent hybridization in both directions and are likely to contribute to the maintenance of species boundaries. Future cross-fertilization studies will hopefully also include the other species pairs of the species complex. Furthermore, the inclusion of other components of prezygotic barriers in future studies would also be very worthwhile, e.g. spatial and temporal segregation, mixis induction and mating behavior.
Data availability
Data are present in the main document and supplementary materials.
References
Bates, D., M. Mächler, B. Bolker & S. Walker, 2014. Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:14065823.
Behm, Jocelyn E., Anthony R. Ives & Janette W. Boughman, 2010. Breakdown in postmating isolation and the collapse of a species pair through hybridization. The American Naturalist 175(1): 11–26.
Carrió, E. & J. Güemes, 2014. The effectiveness of pre- and post-zygotic barriers in avoiding hybridization between two snapdragons (Antirrhinum L.: Plantaginaceae). Botanical Journal of the Linnean Society 176(2): 159–172.
Chin, T. A., C. E. Cáceres & M. E. Cristescu, 2019. The evolution of reproductive isolation in Daphnia. BMC Evolutionary Biology 19(1): 216.
Coughlan, J. M. & D. R. Matute, 2020. The importance of intrinsic postzygotic barriers throughout the speciation process. Philosophical Transactions of the Royal Society b: Biological Sciences 375(1806): 20190533.
Coyne, J. A. & H. A. Orr, 1997. “Patterns of speciation in Drosophila” revisited. Evolution 51(1): 295–303.
de Beauchamp, P. (1965): Classe des rotifères. In: Grassé, P.-P. (ed) Traité de zoologie, T 4, 3 Némathelmintes (Nématodes, Gordiacés), Rotifères, Gastrotriches, Kinorhynques, pp. 1225–1379. Masson et Cie, Paris
de Queiroz, K., 2007. Species concepts and species delimitation. Systematic Biology 56(6): 879–886.
Declerck, S. A. J., A. R. Malo, S. Diehl, D. Waasdorp, K. D. Lemmen, K. Proios & S. Papakostas, 2015. Rapid adaptation of herbivore consumers to nutrient limitation: Eco-evolutionary feedbacks to population demography and resource control. Ecology Letters 18(6): 553–562.
Dobzhansky, T., 1950. Mendelian populations and their evolution. The American Naturalist 84(819): 401–418.
Edmands, S., S. L. Northrup & A. S. Hwang, 2009. Maladapted gene complexes within populations of the intertidal copepod Tigriopus californicus? Evolution 63(8): 2184–2192.
Fontaneto, D., 2014. Molecular phylogenies as a tool to understand diversity in rotifers. International Review of Hydrobiology 99(1–2): 178–187.
García-Morales, A. E. & M. Elías-Gutiérrez, 2013. DNA barcoding of freshwater Rotifera in Mexico: Evidence of cryptic speciation in common rotifers. Molecular Ecology Resources 13(6): 1097–1107.
Gilbert, J. J., 2003. Specificity of crowding response that induces sexuality in the rotifer Brachionus. Limnology and Oceanography 48(3): 1297–1303.
Gilbert, J. J., 2007. Induction of mictic females in the rotifer Brachionus: oocytes of amictic females respond individually to population-density signal only during oogenesis shortly before oviposition. Freshwater Biology 52(8): 1417–1426.
Gilbert, J. J., 2010. Effect of food concentration on the production and viability of resting eggs of the rotifer Brachionus: implications for the timing of sexual reproduction. Freshwater Biology 55(12): 2437–2446.
Gilbert, J. J. & E. J. Walsh, 2005. Brachionus calyciflorus is a species complex: Mating behavior and genetic differentiation among four geographically isolated strains. Hydrobiologia 546(1): 257–265.
Gilman, R. T. & J. E. Behm, 2011. Hybridization, species collapse, and species reemergence after disturbance to premating mechanisms of reproductive isolation. Evolution 65(9): 2592–2605.
Giraud, T., G. Refrégier, M. Le Gac, D. M. de Vienne & M. E. Hood, 2008. Speciation in fungi. Fungal Genetics and Biology 45(6): 791–802.
Gómez, A. & G. Carvalho, 2000. Sex, parthenogenesis and genetic structure of rotifers: microsatellite analysis of contemporary and resting egg bank populations. Molecular Ecology 9(2): 203–214.
Gómez, A. & T. Snell, 1996. Sibling species and cryptic speciation in the Brachionus plicatilis species complex (Rotifera). Journal of Evolutionary Biology 9(6): 953–964.
Gross, B. L. & L. H. Rieseberg, 2005. The ecological genetics of homoploid hybrid speciation. Journal of Heredity 96(3): 241–252.
Guillard, R. R., 1975. Culture of phytoplankton for feeding marine invertebrates Culture of marine invertebrate animals. Springer 29–60
Holzman, R. & C. D. Hulsey, 2017. Mechanical transgressive segregation and the rapid origin of trophic novelty. Scientific Reports 7: 40306.
Hopkins, R., 2013. Reinforcement in plants. New Phytologist 197(4): 1095–1103.
Hwang, D.-S., H.-U. Dahms, H. G. Park & J.-S. Lee, 2013. A new intertidal Brachionus and intrageneric phylogenetic relationships among Brachionus as revealed by allometry and CO1-ITS1 gene analysis. Zoological Studies 52(1): 13.
Jewell, C., A. D. Papineau, R. Freyre & L. C. Moyle, 2012. Patterns of reproductive isolation in Nolana (Chilean bellflower). Evolution 66(8): 2628–2636.
Kagawa, K. & G. Takimoto, 2018. Hybridization can promote adaptive radiation by means of transgressive segregation. Ecology Letters 21(2): 264–274.
Keller, B., J. Wolinska, C. Tellenbach & P. Spaak, 2007. Reproductive isolation keeps hybridizing Daphnia species distinct. Limnology and Oceanography 52(3): 984–991.
Kimpel, D., J. Gockel, G. Gerlach & O. R. P. Bininda-Emonds, 2015. Population structuring in the monogonont rotifer Synchaeta pectinata: High genetic divergence on a small geographical scale. Freshwater Biology 60(7): 1364–1378.
Kordbacheh, A., A. N. Shapiro & E. J. Walsh, 2019 Reproductive isolation, morphological and ecological differentiation among cryptic species of Euchlanis dilatata, with the description of four new species. Hydrobiologia
Lackey, A. C. R. & J. W. Boughman, 2017. Evolution of reproductive isolation in stickleback fish. Evolution 71(2): 357–372.
Lamichhaney, S., F. Han, M. T. Webster, L. Andersson, B. R. Grant & P. R. Grant, 2018. Rapid hybrid speciation in Darwin’s finches. Science 359(6372): 224.
Leliaert, F., H. Verbruggen, P. Vanormelingen, F. Steen, J. M. López-Bautista, G. C. Zuccarello & O. De Clerck, 2014. DNA-based species delimitation in algae. European Journal of Phycology 49(2): 179–196.
Lemmen, K. D., L. Zhou, S. Papakostas & S. A. J. Declerck, 2020 The growth rate hypothesis as a predictive framework for microevolutionary adaptation to selection for high population growth: an experimental test under phosphorus rich and phosphorus poor conditions. bioRxiv:2020.06.14.150649
Li, H., Y. Xi, X. Cheng, X. Xiang, C. Hu & L. Tao, 2008. Sympatric speciation in rotifers: Evidence from molecular phylogenetic relationships and reproductive isolation among Brachionus calyciflorus clones. Acta Zoologica Sinica 51(11): 1099–1128.
Mallet, J., 2005. Hybridization as an invasion of the genome. Trends in Ecology & Evolution 20(5): 229–237.
Mark Welch, D. B., J. L. Mark Welch & M. Meselson, 2008. Evidence for degenerate tetraploidy in bdelloid rotifers. Proceedings of the National Academy of Sciences 105(13): 5145.
Matute, D. R., 2010. Reinforcement of gametic isolation in Drosophila. PLoS Biology 8(3): 1000341.
Mavárez, J. & M. Linares, 2008. Homoploid hybrid speciation in animals. Molecular Ecology 17(19): 4181–4185.
Mayr, E., 1942. Systematics and the origin of species, from the viewpoint of a zoologist, Harvard University Press:
Michaloudi, E., S. Papakostas, G. Stamou, V. Neděla, E. Tihlaříková, W. Zhang & S. A. J. Declerck, 2018. Reverse taxonomy applied to the Brachionus calyciflorus cryptic species complex: Morphometric analysis confirms species delimitations revealed by molecular phylogenetic analysis and allows the (re)description of four species. PLOS ONE 13(9): e0203168.
Montero-Pau, J., A. Gómez & J. Muñoz, 2008. Application of an inexpensive and high-throughput genomic DNA extraction method for the molecular ecology of zooplanktonic diapausing eggs. Limnology and Oceanography: Methods 6(6): 218–222.
Moreno, E., J. M. Conde-Porcuna & A. Gómez, 2017. Barcoding rotifer biodiversity in Mediterranean ponds using diapausing egg banks. Ecology and Evolution 7(13): 4855–4867.
Moyle, L. C., M. S. Olson & P. Tiffin, 2004. Patterns of reproductive isolation in three angiosperm genera. Evolution 58(6): 1195–1208.
Nichols, P., M. J. Genner, C. van Oosterhout, A. Smith, P. Parsons, H. Sungani, J. Swanstrom & D. A. Joyce, 2015. Secondary contact seeds phenotypic novelty in cichlid fishes. Proceedings of the Royal Society B: Biological Sciences 282(1798): 20142272.
Obertegger, U., G. Flaim & D. Fontaneto, 2014. Cryptic diversity within the rotifer Polyarthra dolichoptera along an altitudinal gradient. Freshwater Biology 59(11): 2413–2427.
Ostevik, K. L., R. L. Andrew, S. P. Otto & L. H. Rieseberg, 2016. Multiple reproductive barriers separate recently diverged sunflower ecotypes. Evolution 70(10): 2322–2335.
Papakostas, S., E. Michaloudi, K. Proios, M. Brehm, L. Verhage, J. Rota, C. Peña, G. Stamou, V. L. Pritchard, D. Fontaneto & S. A. J. Declerck, 2016. Integrative taxonomy recognizes evolutionary units despite widespread mitonuclear discordance: Evidence from a rotifer cryptic species complex. Systematic Biology 65(3): 508–524.
Paraskevopoulou, S., A. B. Dennis, G. Weithoff & R. Tiedemann, 2020. Temperature-dependent life history and transcriptomic responses in heat-tolerant versus heat-sensitive Brachionus rotifers. Scientific Reports 10(1): 13281.
Paraskevopoulou, S., R. Tiedemann & G. Weithoff, 2018. Differential response to heat stress among evolutionary lineages of an aquatic invertebrate species complex. Biology Letters 14(11)
Piscia, R., P. Guilizzoni, D. Fontaneto, D. A. L. Vignati, P. G. Appleby & M. Manca, 2012. Dynamics of rotifer and cladoceran resting stages during copper pollution and recovery in a subalpine lake. Annales De Limnologie - International Journal of Limnology 48(2): 151–160.
Rheindt, F. E., M. K. Fujita, P. R. Wilton & S. V. Edwards, 2014. Introgression and phenotypic assimilation in Zimmerius Flycatchers (Tyrannidae): Population genetic and phylogenetic inferences from genome-wide SNPs. Systematic Biology 63(2): 134–152.
Ruttner-Kolisko, A., 1969. Kreuzungsexperimente zwischen Brachionus urceolaris und Brachionus quadridentatus, ein beitrag zur fortpflanzungsbiologie der heterogonen Rotatoria. Arch Hydrobiol 65: 397–412.
Sánchez-Guillén, R. A., M. Wellenreuther & A. Cordero Rivera, 2012. Strong asymmetry in the relative strengths of prezygotic and postzygotic barriers between two damselfly sister species. Evolution 66(3): 690–707.
Sánchez-Guillén, R. A., A. Córdoba-Aguilar, A. Cordero-Rivera & M. Wellenreuther, 2014. Rapid evolution of prezygotic barriers in non-territorial damselflies. Biological Journal of the Linnean Society 113(2): 485–496.
Schröder, T. & E. Walsh, 2007. Cryptic speciation in the cosmopolitan Epiphanes senta complex (Monogononta, Rotifera) with the description of new species. Hydrobiologia 593(1): 129–140.
Schwenk, K., N. Brede & B. Streit, 2008. Introduction Extent, processes and evolutionary impact of interspecific hybridization in animals. Philosophical Transactions of the Royal Society B Biological Sciences 363(1505): 2805–2811.
Seehausen, O. L. E., G. Takimoto, D. Roy & J. Jokela, 2008. Speciation reversal and biodiversity dynamics with hybridization in changing environments. Molecular Ecology 17(1): 30–44.
Serra, M., E. M. García-Roger, R. Ortells & M. J. Carmona, 2019. Cyclically parthenogenetic rotifers and the theories of population and evolutionary ecology. Limnetica 38(1): 67–93.
Snell, T. W., 2017. Analysis of proteins in conditioned medium that trigger monogonont rotifer mictic reproduction. Hydrobiologia 796(1): 245–253.
Snell, T. W. & M. Childress, 1987. Aging and loss of fertility in male and female Brachionus plicatilis (Rotifera). International Journal of Invertebrate Reproduction and Development 12(1): 103–110.
Snell, T. W. & C.-P. Stelzer, 2005. Removal of surface glycoproteins and transfer among Brachionus species. Hydrobiologia 546(1): 267–274.
Snell, T., R. Rico-Martinez, L. Kelly & T. Battle, 1995. Identification of a sex pheromone from a rotifer. Marine Biology 123(2): 347–353.
Snell, T. W., J. Kubanek, W. Carter, A. B. Payne, J. Kim, M. K. Hicks & C.-P. Stelzer, 2006. A protein signal triggers sexual reproduction in Brachionus plicatilis (Rotifera). Marine Biology 149(4): 763–773.
Snell, T. W., J. Kim, E. Zelaya & R. Resop, 2007. Mate choice and sexual conflict in Brachionus plicatilis (Rotifera). Hydrobiologia 593(1): 151–157.
Staats, M. & P. v. Baarlen & J. A. L. v. Kan, 2004. Molecular phylogeny of the plant pathogenic genus Botrytis and the evolution of host specificity. Molecular Biology and Evolution 22(2): 333–346.
Stelkens, R. B., K. A. Young & O. Seehausen, 2010. The accumulation of reproductive incompatibilities in African cichlid fish. Evolution 64(3): 617–633.
Stelzer, C. & T. Snell, 2003. Induction of sexual reproduction in Brachionus plicatilis (Monogononta, Rotifera) by a density-dependent chemical cue. Limnology and Oceanography 48(2): 939–943.
Vallejo-Marín, M., R. J. A. Buggs, A. M. Cooley & J. R. Puzey, 2015. Speciation by genome duplication: Repeated origins and genomic composition of the recently formed allopolyploid species Mimulus peregrinus. Evolution 69(6): 1487–1500.
Whitney, K. D., J. R. Ahern, L. G. Campbell, L. P. Albert & M. S. King, 2010. Patterns of hybridization in plants. Perspectives in Plant Ecology, Evolution and Systematics 12(3): 175–182.
Wu, H., Z. Ma, M.-M. Wang, A.-L. Qin, J.-H. Ran & X.-Q. Wang, 2016. A high frequency of allopolyploid speciation in the gymnospermous genus Ephedra and its possible association with some biological and ecological features. Molecular Ecology 25(5): 1192–1210.
Xiang, X., Y. Xi, X. Wen, G. Zhang, J. Wang & K. Hu, 2011a. Genetic differentiation and phylogeographical structure of the Brachionus calyciflorus complex in eastern China. Molecular Ecology 20(14): 3027–3044.
Xiang, X., Y. Xi, X. Wen, G. Zhang, J. Wang & K. Hu, 2011b. Patterns and processes in the genetic differentiation of the Brachionus calyciflorus complex, a passively dispersing freshwater zooplankton. Molecular Phylogenetics and Evolution 59(2): 386–398.
Yin, M. B., A. Petrusek, J. Seda & J. Wolinska, 2012. Fine-scale temporal and spatial variation of taxon and clonal structure in the Daphnia longispina hybrid complex in heterogeneous environments. BMC Evolutionary Biology 12: 12.
Zhang, W. & S. A. J. Declerck, 2022. Intrinsic postzygotic barriers constrain cross-fertilisation between two hybridising sibling rotifer species of the Brachionus calyciflorus species complex. Freshwater Biology 67: 240–249.
Zhang, Y., A. Zhou, Y. Xi, Q. Sun, L. Ning, P. Xie, X. Wen & X. Xiang, 2018. Temporal patterns and processes of genetic differentiation of the Brachionus calyciflorus (Rotifera) complex in a subtropical shallow lake. Hydrobiologia 807(1): 313–331.
Zhang, W., K. D. Lemmen, L. Zhou, S. Papakostas & S. A. J. Declerck, 2019. Patterns of differentiation in the life history and demography of four recently described species of the Brachionus calyciflorus cryptic species complex. Freshwater Biology 64(11): 1994–2005.
Acknowledgements
We wish to thank Dennis Waasdorp and Michaela Brehm for their help with field and lab work. We also thank Spiros Papakostas for his advice on molecular analysis and comments on an early draft of this manuscript.
Funding
This work was supported by a PhD Grant of the China Scholarship Council (CSC), 201508360096 to WZ.
Author information
Authors and Affiliations
Contributions
WZ and SD designed this study, WZ performed the experiment, WZ and SD carried out the statistical analyses and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare we have no conflict of interest.
Additional information
Handling Editor: Diego Fontaneto
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, W., Declerck, S.A.J. Reduced fertilization constitutes an important prezygotic reproductive barrier between two sibling species of the hybridizing Brachionus calyciflorus species complex. Hydrobiologia 849, 1701–1711 (2022). https://doi.org/10.1007/s10750-022-04814-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04814-y