Abstract
The diversion of sewage inputs in the mid-1970s led to an order of magnitude reduction in nutrient loading to Lake Vesijärvi, southern Finland. After the diversion, nutrient concentrations declined, consistent with a simple dilution model, and by the mid-1990s the chlorophyll concentration was reduced by 80%. The favourable development was supported by a 5-year mass removal of planktivorous and benthivorous fish and the stocking of predatory pikeperch (Sander lucioperca (L.)), although the exact mechanisms behind their effects remain obscure. Starting in 2010, oxygen-rich water from the top of the water column was pumped to the deepest parts of the lake, resulting in high deepwater oxygen concentration in winter. In summer, hypoxic or even anoxic conditions could not be avoided, but the duration of the anoxic period was markedly shortened. Because nitrate was never depleted, leaching of total nitrogen from the sediment was reduced and the same was also true for total phosphorus, but only in winter. The oxygenation stabilized deepwater nutrient concentrations to a low level, but this was not reflected in the epilimnetic total nutrient concentration or in a further decrease in the chlorophyll concentration.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
The recreational and fisheries value of eutrophic lakes is often damaged by an excessive abundance of phytoplankton. To alleviate the problems, a large variety of physical, chemical and biological methods have been applied in widely different environments. Specific local conditions and available financing determine what method, or combination of methods, and time span of the measures are possible in practice. A reduction in external loading is the basic method to combat eutrophication, but the recovery may involve considerable delays. To speed up the process, various in-lake methods have been used. The mainstream methods may be classified into physical methods, which target improvement of deepwater oxygen conditions, chemical, which target a reduction in the availability of nutrients in water, and biological, which target shifting the primary control of phytoplankton from bottom-up to top-down.
Oxygenation of deep lake water has been widely used in restoration projects all over the world (Beutel & Horne, 1999; Bormans et al., 2016). It is based on the theory that when the surface sediment becomes anoxic, ferric iron is reduced to ferrous iron, which releases the bound phosphate (Einsele, 1936; Mortimer, 1941). Consequently, oxygen depletion leads to increased nutrient concentrations, which enhance primary production with its associated problems in water quality. However, the opposite conclusion, that anoxia is the consequence rather than the cause of elevated nutrient concentrations (Gächter & Wehrli, 1998; Hupfer & Lewandowski, 2008; Salmi et al., 2014), has increasingly resonated with scientists. Indeed, the ferric iron—phosphorus (P) theory has long been proposed to be an oversimplification (Lee et al., 1977), but only rather recently have critical views been taken more seriously (Nygrén et al., 2017). With full lift aeration or pumping of epilimnetic water into the hypolimnion it is difficult to increase hypolimnetic oxygen concentrations above 2 g m−3 in late summer (Liboriussen et al., 2009; Salmi et al., 2014; Kuha et al., 2016) and water temperature is increased (Beutel & Horne, 1999; Bryant et al., 2011; Salmi et al., 2014), accelerating hypolimnetic decomposition. The use of pure oxygen instead of air provides much higher oxygenation efficiency (Beutel & Horne, 1999), but its running costs are high.
Biomanipulation by fish removal has also been widely used in attempts to limit problems following phytoplankton blooms. The basic idea is simple and repeatedly validated experimentally: when predation on grazers is low, zooplankton are able to control phytoplankton biomass so that water quality is improved. Similar to oxygenation, biomanipulation has often been taken as too simple and the results have been mixed (Hansson et al., 1998; Drenner & Hambright, 2002; Mehner, 2010; Bernes et al., 2015). The interpretation of the results is prone to many kinds of pitfalls, which makes conclusions challenging. In particular, the time span of studies is often too short so that all effects may not have had time to be realized. Various measures are also typically taken simultaneously, making it impossible to differentiate between their individual effects. Moreover, as pointed out by Horppila et al. (1998), when many indirect effects with various time spans are coupled with stochastic variation in weather, the outcome often becomes impossible to interpret. Finally, because the failures of the measures likely have been under-reported in the literature and research reports may overemphasize positive aspects at the expense of negative ones, the overall conclusions may also be biased to an unknown extent.
Multifaceted studies with a long time span improve possibilities to interpret the results but still the same problems largely exist, and due to economic reasons, such approaches are rare. Replication of lake-scale results is virtually impossible and reference lakes are often lacking. Consequently, final conclusions can generally be approached only after the analysis of many studies covering various methods and different lake types. The present documentation of the long-term efforts to improve the water quality of Lake Vesijärvi adds to the knowledge contributing to the synthesis of different remediation methods. The restoration history of Lake Vesijärvi has the advantages of a long time span, a wide variety of measurements and to some extent also the possibility to scrutinize the effects of the measures against the results of another basin of the same lake and a neighbouring lake.
Study lake and methods
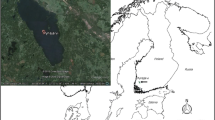
Lake Vesijärvi (Fig. 1), in southern Finland, is a seepage lake located between the Salpausselkä ridges to the north and south. Its Enonselkä basin, which formerly received the waste water of the city of Lahti, is characterized by a rather long retention time of 5.6 years. Water from the Enonselkä basin flows to the Kajaanselkä basin, whose residence time is less than half that of the Enonselkä basin (Table 1).
The water quality of Lake Vesijärvi deteriorated mainly due to municipal waste water of the nearby city of Lahti. In the 1970s, sewage water of 60,000 inhabitants was discharged almost untreated into the Enonselkä basin, which led to a loss of its value for local citizens. The first step in the improvement of the water quality of the lake was taken when a waste-water treatment plant was taken into service and its effluent led away from the catchment area of Lake Vesijärvi. In 1975, the effluent load was cut to a third and additional cuts in the following year decreased the original external load of roughly 2 g m−2 P year−1 to Lake Vesijärvi by an order of magnitude (Keto & Sammalkorpi, 1988). In the 1980s, the diversion of industrial waste water further reduced the nutrient load by ~ 15% (Horppila et al., 1998). In spite of the radical decrease in external load, after less than 10 years the Enonselkä basin was plagued by mass blooms of cyanobacteria throughout the year, which led to attempts to reinforce the recovery of the basin by various methods, as outlined by Keto & Sammalkorpi (1988). Many approaches were tested at different scales, but the main ones were a further reduction in the external load, oxygenation of the hypolimnion, as well as biomanipulation by removal of planktivorous and benthivorous fish and stocking of predatory fish. The results until the mid-2000s have been summarized by Keto et al. (2005) and the later deepwater oxygenation period by Salmi et al. (2014).
To increase oxygen concentration in deep water, in 1979–1984, a pump station was installed at the deepest part of the Enonselkä basin and it was operated in winter. In 1983, the number of pumps was increased to three and they were operated also in summer. Water was pumped from 1 m depth to the hypolimnion 8–10 m above the sediment to avoid resuspension. For simplicity in the following, the term ‘oxygenation’ is used to describe the introduction of oxic epilimnetic water into the hypolimnion. From 2010, the same approach (Lappalainen, 1994; Bendtsen et al., 2013) was applied at a large scale with eight 2.5 kW pump stations and one 1.5 kW station located above the deep water of the Enonselkä basin (Fig. 1b). To save energy, in some winters only part of the pumps were operated (Table 2). The detailed operation times of the pumps in summer are given by Ruuhijärvi et al. (2020).
The mass removal of planktivorous and benthivorous fish from the Enonselkä basin was performed in May–August (Keto & Tallberg, 2000) by trawling over the areas deeper than 10 m (in 1989) or 5–6 m (in 1990–1993). From 1994, trawling was replaced by fyke net fishing in spring–summer and seine fishing in autumn. Weather data were obtained from the Lahti Laune weather station of the Finnish Meteorological Institute. Chemical and physical determinations were made in the laboratories of local and regional authorities using standard methods similar to those described by Horppila et al. (1998). Most results are available from the service of the Finnish Environment Institute (27 February 2020, https://www.syke.fi/en-US/Open_information/Open_web_services). Some total P (TP) and total nitrogen (TN) results were taken from Keto (1982). Special attention was paid to the results of late summer, because it is then that water quality problems are typically emphasized.
The graphics as well as the correlation and regression statistics calculations of the results were made with Microsoft Excel 2016. Theoretical dilution curves of waste water since its diversion were estimated from the initial and final concentrations of the time series and from the 5.6-year residence time (r) according to the formula
where y = concentration n years after the sewage diversion, c0 = initial concentration and ct = final concentration (estimated from the mean of the last 15 years corrected for the dilution during that time).
From 1988, atmospheric deposition of inorganic N was measured by the Finnish Meteorological Institute within a pristine forest area at a distance of ~ 40 km from Lake Vesijärvi. Atmospheric deposition of P on the lake surface was estimated at two stations near the shore of Lake Vesijärvi in 2008–2009 (Autio & Malin, unpublished report). The statistics of fertilizers used in agriculture were compiled by the Natural Resources Institute Finland (27 February 2020, http://statdb.luke.fi/PXWeb/pxweb/fi/LUKE/LUKE__08%20Indikaattorit__06%20Ympäristö__12%20Typpi-%20ja%20fosforitase/02_Kasviravinteiden_myynti.px/?rxid=e119108e-bd95-43bf-b02c-a5f4ff9eef72).
Results
In June–August 1975–2018, air temperature (Fig. 2) increased on average by 2.1°C (SD 0.9°C, where SD is standard deviation of the mean). However, during the most intensive fish removal period (Fig. 3), mean summer temperature consistently decreased by ~ 2.5°C. Annual precipitation did not change significantly, but there were different phases. From 1977 to 1985, the interannual variation was high, while during the following 13 years there was a rather smooth ~ 160 mm decrease (SD 36 mm year−1 after the removal of the variation explained by linear regression for that period). After 1998, the interannual variation (SD 103 mm year−1) returned to the level prevailing at the beginning of the study.
After the diversion of waste water, some other components of external nutrient load to Lake Vesijärvi also decreased. Coincident with the intensive fish removal, after 1988 N deposition halved within 10 years (Fig. 4), but later its decrease was slower. Atmospheric deposition of P on the lake surface in 2008–2009 (Autio & Malin, unpublished report) was 0.02 g P m−2 year−1. If the same decrease as observed for N is applied for P, then it would mean that atmospheric deposition was about 4% of the total load of P to the Enonselkä basin in 2008–2009, corresponding to about 12% by 1988. Between 1988 and 1997, the amount of P fertilizer used in agriculture in Finland decreased by 63% and after that 18% more, while the respective decreases of N fertilizer were 12% and 14%.
Temperature and oxygen in deep water
Before the installation of the pump stations in the Enonselkä basin to oxygenate the hypolimnion, the maximum temperature at 29 m depth before the autumnal overturn varied between 9 and 15°C without any significant trend. Oxygen concentration was always < 1.5 g m−3 and often depleted (concentration before oxygenation < 0.4 g m−3 in 25 of 33 cases) at the end of the summer stratification (Fig. 5). The longest period of consistent anoxia at 29 m in summer occurred in the mid-2000s. Due to variable weather conditions, sometimes significant deepening of the thermocline was observed already before the sampling in August and then the results of July were used to approximate the distance between the surface and the depth where hypoxic conditions started (Fig. 6). Following the decreasing trend in mean summer temperature during the intensive fish removal (Fig. 2), the depth of 2 g m−3 oxygen concentration shifted from 10–15 m depth to about 20 m. In 1997–2009, the stratification of oxygen was again similar to that before 1986. Because water temperature explained little (1977–2009 R2 < 0.01 in late summer) of the variation in deepwater oxygen concentration, wind episodes in early summer must have played a dominant role in the variation of hypolimnetic oxygen conditions. In the Kajaanselkä basin, oxygen concentration at 36–40 m depth was never depleted. Its increasing concentration from the end of the1980s (Fig. 6) was consistent with the decreasing thickness of the hypoxic hypolimnion in the Enonselkä basin (Fig. 5), corroborating enhancement of mixing by weather conditions in both basins. The oxygenation increased the upper range of deepwater temperature in the Enonselkä basin to 18°C (Fig. 7), but despite the generally increasing oxygen concentration, the hypolimnion remained hypoxic or sometimes even anoxic in late summer.
Vertical distribution of oxygen concentration in July–August of 1975–2018 before the beginning of autumnal deepening of the epilimnion. Each distribution is shifted by 5 g m−3 to the right; the oxygen scale is in 5 g m−3 steps and the years are shown at zero concentration; black lines with dots represent the depth of the 2 g m−3 concentration. Thick red dashed lines indicate the intensive fish removal (upper panel) and oxygenation periods (lower panel)
In winter, the temperature at 29 m in the Enonselkä basin varied between 2.8 and 4.5°C before the oxygenation, which partly explains the higher oxygen concentration and less frequent anoxia than in summer. Temperatures sometimes exceeding the maximum density temperature of water near the bottom indicated chemical stratification, which is an important factor potentially delaying and shortening the spring overturn. Oxygenation of the Enonselkä basin after 2009 decreased the deepwater winter temperature by ~ 2°C and increased oxygen concentration to 7.6–11.3 g m−3. Even the less powerful pump located at the sampling station in the early 1980s achieved ~ 7 g m−3 oxygen concentration in the deep water. In the Kajaanselkä basin, deepwater oxygen concentration was also generally hypoxic, but in spite of the greater depth it was never depleted.
Development of nutrient concentrations in the uppermost water layers
In May–June, TP and TN concentrations at 1 m depth in the Enonselkä basin decreased more or less in parallel after the diversion of waste water (Fig. 8a, b). In July–August, the concentration of TP was consistently higher than in May–June, but the difference narrowed from 1.6 times at the beginning of the study period to 1.2 times in 2018. Instead, there was no significant difference in the TN concentration between early and late summer (Sign test z = 0, P > 0.10, P = probability). The decreases in TP and TN concentrations were roughly explained by the dilution model (R2 = 0.69 and 0.39 in May–June and 0.64 and 0.59 in July–August, respectively, R2 = coefficient of determination). In July–August, both TP and TN developed initially as predicted by the model, but in the mid-1980s, when exceptionally high pH values of up to 9.5 were measured (Fig. 8f), a positive anomaly in relation to the dilution curve developed. The concentrations of TP and TN started to decrease after 1987 with a particularly marked step downward between 1992 and 1993. The concentrations of both nutrients after the late 1990s roughly followed the dilution curve and made a single cluster without any temporal trend (Fig. 9a), indicating that a rather stable state had been reached. The final concentrations were about 0.03 g m−3 for TP and 0.45 g m−3 for TN. The TN:TP mass ratio remained rather similar throughout the study period. No correlation was found between TP and the depth where oxygen concentration reached 2 g m−3. With the exception of TP in the late 1970s, the concentrations of TP and TN in the Kajaanselkä basin were similar in early and late summer (Fig. 8a). The results in the Enonselkä basin were an average 2.2 (SD = 0.5) and 1.4 (SD = 0.2) times, respectively, higher than those of the Kajaanselkä basin. In the time series of the Kajaanselkä basin, there was no sign of the sudden decrease in concentration between 1992 and 1993 found in the Enonselkä basin, but the relationship between the TP values in both basins stabilized after about 1993 (Fig. 8e). Compared with the years 2001–2009, the oxygenation during 2010–2018 did not cause a significant difference (Mann–Whitney U test) in TP and TN concentration of the Enonselkä basin.
a Three-year moving averages of total phosphorus, b total nitrogen, c chlorophyll a and d Secchi depth of the Enonselkä (black lines) and Kajaanselkä (orange lines) basins in May–June (thin lines) and July–August (bold lines); e comparison of the results of the Enonselkä and Kajaanselkä basins for mean concentration of TP (blue lines) and Secchi depth (green lines) in July–August (thick lines) with their superimposed counterparts in May–June (thin lines); f variation in pH of the Enonselkä basin at 1 m depth in summer. Dotted lines—development of concentration in the Enonselkä basin fitted to the dilution model; shaded vertical bars—intensive fish removal period; circles—mean values in July–August
Relationship between total phosphorus and total nitrogen in the Enonselkä and Kajaanselkä basins in 1977–2018. Dashed line—Redfield N:P mass ratio of 7:1; upper dotted line—20:1; lower dotted line—3:1; the time series of the period 1977–1988 is shown by solid lines and numbers denote the last two digits of the sampling years
In March to early April, total nutrient concentrations at 1 m depth in the Enonselkä basin were occasionally very high between 1984 and 1989 (Fig. 10), and the same happened once in the Kajaanselkä basin. The high concentrations at the depth nearest to the surface at the same time suggest that they were due to meltwaters, which depend on the timing of the late winter sampling. Because of these irregularities, the data from 15 m depth in the Enonselkä basin were used to fit the dilution curve. However, although the concentrations at 15 m depth were slightly higher, the overall development of the nutrient concentrations at 1 m and 15 m depths during winter was similar. The dilution model explained most of the variation (R2 = 0.87) of TP at 15 m depth, but the respective value for TN (R2 = 0.50) was slightly lower than in summer. At the end of the study, the concentrations of TP and TN in the Enonselkä basin were about 1.4 times higher than those in the Kajaanselkä basin.
Other long time series of the lakes in the vicinity of Lake Vesijärvi were available only from more oligotrophic Lake Pääjärvi (area 13 km2, depth 85 m), which is located 6 km west of Lake Vesijärvi. The land use of its catchment area is comparable with Lake Vesijärvi: forests 57% (Lake Vesijärvi 60%) and agricultural fields 18% (23%). Between 1981 and 2018, epilimnetic TP concentration of Lake Pääjärvi decreased (Fig. 11) by roughly 25% (R2 = 0.21, P = 0.02), while the respective decrease in the Kajaanselkä basin was roughly 30%. Thus, in addition to possible regional improvement, decreased TP concentration of the Kajaanselkä basin probably also reflected decreased nutrient inflow from the Enonselkä basin.
Development of nutrient concentrations in deep water
In July–August, the deepwater concentrations of TP and TN in the Enonselkä basin were an order of magnitude higher than in the epilimnion (Fig. 12a, b), but between 1977 and 2009 their rates of decrease were faster, roughly 95% vs 40% in TP and 75% vs 50% in TN. Following the most rapid recovery, between 1998 and 2009 the concentrations of TP and TN were on average 4.7 and 2.6 times higher, respectively, than in the Kajaanselkä basin. Coefficient of variation (CV) was much higher in the Enonselkä than Kajaanselkä basin, 67% of the mean vs 18% for TP and 22% vs 8% for TN. In March–April, the concentrations of TP and TN only very weakly followed the dilution trend. Instead, they showed irregular alternation between a rather stable low level, typically slightly lower than in summer, and up to an order of magnitude higher concentrations (Fig. 12c, d), which occurred in the years when oxygen concentration decreased below 2 g m−3. It was notable that low and high concentrations often occurred in successive years. The burst of nutrients from the sediment was particularly striking in 1986 and this event also extended over several years. In the Kajaanselkä basin, TP and TN concentrations closely corresponded to the lowest levels of the Enonselkä basin. The high difference in TP and TN concentrations between the basins in July–August despite a negligible difference in March–April probably reflected higher primary production and sedimentation of phytoplankton in the Enonselkä basin. The only detailed vertical series of samples from the winter of 1990 showed an extremely steep gradient of 1.6 g m−3 in TP within a 1.7 m depth interval near the sediment. Consequently, on the basis of these results and bathymetric data of the Enonselkä basin, TP in water deeper than 15 m contributed ~ 6% to the TP concentration of the whole water mass of the Enonselkä basin. The oxygenation stabilized deepwater TP concentration of the Enonselkä basin in summer to a level corresponding to the lowest earlier results, but the average TN concentration was roughly halved.
The relationship between oxygen and the main nutrients in deep water was close to hyperbolic particularly in winter. When oxygen decreased below 2 g m−3, low as well as high nutrient concentrations were found both in summer and in winter, but concentrations were always low at higher oxygen levels. The respective relationship to nitrate was even more strongly hyperbolic (Fig. 13), showing that after the depletion of oxygen, P release from the sediment was accelerated only if nitrate was also exhausted.
The development of the TN:TP ratio was more dynamic in the deep water than in the upper layers (Fig. 9). At the beginning of the recovery of the Enonselkä basin, the TN:TP mass ratio was less than 3 in winter, well below the Redfield ratio, indicating N deficiency, but along with the decrease in total nutrients in the early 1980s it shifted to higher than the Redfield ratio, indicating P deficiency of phytoplankton. However, at the time of the increased nutrient release from the sediment in 1986 (Fig. 9), the TN:TP ratio returned to about 3. During the intensive fish removal, a shift back towards the ratio prevailing in 1981–1984 occurred. Although the TN:TP ratio finally stabilized at about 20, in the years 1996, 2003 and 2009 with temporarily increased nutrient concentrations, lower TN:TP ratios were again observed. In late summer, TP and TN concentrations (with r = 0.80) as well as their ratio developed similarly, but the changes were smaller and the variation was higher than in winter. In winter, the oxygenation did not affect the TN:TP ratio, but in summer it shifted close to the Redfield ratio. Shutting down the pump stations for the summer of 2018 increased the respective TN:TP ratio again to about 20.
Chlorophyll a
The chlorophyll concentration of the Enonselkä basin was higher in late than in early summer, but after the intensive fish removal the difference disappeared (Fig. 8c). The dilution model poorly described the chlorophyll results in July–August, but its application pointed to interesting anomalies in relation to the biomanipulation. The concentrations of TP and TN as well as chlorophyll began to decrease after the diversion of sewage (Fig. 8a–c), but almost 10 years later the favourable development was interrupted and the concentration remained around 30 mg m−3 until the end of 1988. During the intensive fish removal years and 4 years following, the concentration of chlorophyll decreased about 85% and reached about a 30% lower level than predicted by the dilution curve. At the same time, its variation in relation to its respective trend line was much lower than before. After 1997, the concentration of chlorophyll started to increase and was an average of ~ 20% of that at the time of waste-water diversion. The oxygenation had no detectable effect on chlorophyll concentration.
The overall trend in chlorophyll concentration in the Kajaanselkä basin (Fig. 8c) was similar to that in the Enonselkä basin in July–August (R2 = 0.46, P < 0.001) but at a ~ 50% lower level, which closely corresponded to the respective relationship in epilimnetic TP concentration. The chlorophyll concentration of the Enonselkä basin was nearest to that in the Kajaanselkä basin soon after the fish removal, suggesting that its effect was delayed. The decrease in chlorophyll concentration as well as the trend in the chlorophyll:TP ratio in the Kajaanselkä basin were not statistically significant. However, the chlorophyll:TP ratio in the Kajaanselkä basin correlated significantly with that in the Enonselkä basin (r = 0.52, P < 0.01). Because the fish removal of the Kajaanselkä basin started only in 1997 and catches were small (Fig. 3), these hardly affected its water quality. In nearby Lake Pääjärvi, no significant trend was found in chlorophyll or the chlorophyll:TP ratio from 1980 (Fig. 11).
Before the end of the intensive fish removal, the ratio between chlorophyll and the TP concentration of the Enonselkä basin in July–August (Fig. 14) mostly indicated the dominance of small herbivorous zooplankton (Mazumder, 1994), but later until the early 2000s there was a marked shift towards values indicating the dominance of large herbivores, suggesting an improvement in water quality. In the 2000s, the variation in the chlorophyll:TP ratio markedly increased, remaining high throughout the oxygenation years. The mean chlorophyll:TP ratio between 1998 and 2009 was not significantly different from that between 2010 and 2018 (Mann–Whitney U test, P = 0.32), indicating a negligible effect of oxygenation. While the chlorophyll:TP ratio of the Enonselkä basin only temporarily indicated the dominance of large zooplankton, in the Kajaanselkä basin the change was permanent.
Interannual variation in the relationship between mean chlorophyll and total phosphorus in the Enonselkä and Kajaanselkä basins in July–August. The regression lines suggested by Mazumder (1994) indicating the dominance of large (dotted line) and small (solid line) zooplankton are shown for comparison. Thick lines show the time sequence of the years between 1989 and 1997
Secchi depth
During the fish removal, the Secchi depth of the Enonselkä basin increased significantly (Mann–Whitney U test, P < 0.01) from about 1.5 m to 2.5–3 m in 1994–1997. Between 1988 and 1998, the variation between successive years was remarkably low and at the end of this period the values were at their lowest in relation to the Kajaanselkä basin (Fig. 8e). Similar to chlorophyll during the last 15 years, the variation in the Secchi depth of the Enonselkä basin was high. In the Kajaanselkä basin, the Secchi depth was on average 1.4 m higher than that in the Enonselkä basin both in early and late summer and their time courses were quite similar (Fig. 8d).
Discussion
The dilution of waste water formerly discharged into the Enonselkä basin was the main reason for its recovery. This conclusion was strongly supported by the high agreement between the observed total nutrient concentrations and those of the respective dilution curves in winter (Fig. 10), when conditions under the ice are undisturbed by weather, primary producers and inflow, and thermal as well as chemical stratification separate the shallow and deepwater layers from each other (Salmi et al., 2014). Although the dilution of nutrients varied interannually depending on the variation in hydrology, internal loading (Søndergaard et al., 1993; Jilbert et al., this issue), atmospheric deposition and the use of fertilizers in agriculture (Fig. 4), the simple application of the average residence time to calculate the dilution provided a useful background for the interpretation of the results. Because agriculture is responsible for ~ 40% of the total P load to the Enonselkä basin, its ~ 80% decrease between 1988 and 1997 means a dramatic change in potential nutrient resources for phytoplankton. However, because P is tightly bound to the soil, its leaching is strongly delayed (Carpenter, 2005). Accordingly, the P load from the most important inflowing streams and brooks as well as from urban run-off to Lake Vesijärvi (unpublished results of Lahti Environmental Services) decreased only ~ 14% between 1987 and 2015.
The oxygenation of the Enonselkä basin in summer delayed the depletion of hypolimnetic oxygen, but it also decreased the temperature gradient between the epi- and hypolimnion, which advanced the beginning of the autumnal overturn. Consequently, the time was too short to deplete nitrate (Fig. 13) and redox potential remained high enough to keep P in the sediment (Andersen, 1982). On the other hand, the intensified coupling of nitrification and denitrification in deep water due to the oxygenation (Beutel, 2006; Salmi et al., 2014; Holmroos et al., 2016) was evidently responsible for the decreased TN concentration (Fig. 12b, d). In spite of the reduced leaching of both TP and TN from the deepwater sediment, the effect of the oxygenation in the epilimnion remained undetectable as also found elsewhere (Liboriussen et al., 2009; Kuha et al., 2016). Elevated temperature increased the mineralization of organic matter (Salmi et al., 2014; Niemistö et al., 2020) and fewer nutrients were probably buried in the sediment. Originally it was also hoped that the oxygen consumption of the sediment would gradually decrease, but in agreement with Engstrom & Wright (2002) who found no loss of organic sediment in five lakes aerated/circulated for 8 to 18 years, the results did not support that (Fig. 5).
In their review, Preece et al. (2019) concluded that oxygenation of the hypolimnion by oxygen gas can be an important tool ‘when used properly’. Such a conclusion is certainly valid from the perspective of deep water and particularly if oxygen gas is applied. Pumping of epilimnetic water into the hypolimnion is more economical, but its advantages are limited by consequent warming of the hypolimnion. The greatly improved oxygen conditions in deep water and smaller nutrient leakage from the sediment of the Enonselkä basin after the oxygenation agree with the literature (Gächter & Wehrli, 1998; Liboriussen et al., 2009). The absence of a detectable effect on phytoplankton, an adverse influence on cold water fish and increased oxygen consumption in the hypolimnion as well as enhanced recycling of nutrients in the whole water body (Niemistö et al. 2020) did also not support the feasibility of the oxygenation. Finally, because the only extreme burst of nutrients from the sediment happened 30 years ago and the dilution of the waste water as well as the decreases in external loading have been great, precautionary oxygenation is no longer justified.
A new stable TP level in the Enonselkä basin was reached 10–15 years after the sewage diversion as also found by Jeppesen et al. (2005) in 35 subtropical and temperate lakes of various size, depth and trophic state. Instead, for TN they found stabilization times of < 5 years, while in the Enonselkä basin the stabilization time was similar to that of TP. This contrast may arise from the fact that in the Enonselkä basin, as in many other Finnish lakes (e.g. Sarvala et al., this issue), TN levels reflect dinitrogen fixation, which closely follows TP concentration. In the data of Jeppesen et al. (2005), nutrient concentrations are much higher and this link is weaker.
The internal loading of nutrients released from anoxic deepwater sediment has been suggested to be the main P load to many lakes (Nürnberg, 2009), but it has been questioned in the Enonselkä basin (Salmi et al., 2014). In a study covering a comprehensive set of lakes, Tammeorg et al. (2017) also demonstrated that a hypolimnetic oxygen deficit has minor importance for water quality. However, the longer time series of the Enonselkä basin in the present study suggests that in the mid-1980s a combination of many factors led to accelerated internal nutrient loading. Its prerequisites probably included a short duration of spring overturn (not measured), decreasing precipitation leading to decreasing nutrient loading (Fig. 2), and high thickness of the anoxic hypolimnion (Fig. 6). The latter might have shoaled the metalimnion to so shallow water that internal seiches (MacIntyre et al., 1999) strengthened the flux of nutrients from the anoxic hypolimnion to the epilimnion. Another or parallel factor may have been the ebullition of gases (Ohle, 1978), which can abruptly increase the flux of nutrients from the sediment. Hydroacoustic studies have indicated a marked decrease in ebullition compared with that in the 1990s (Ruuhijärvi et al., this issue). The nutrient pulse from the sediment after the middle of the 1980s probably initiated the development of the observed cyanobacteria blooms (Horppila et al., 1998), which elevated pH to such a level (Andersen, 1975) that leaching of P from the epilimnetic sediment increased. In routine samples typically taken before noon, pH values remained below 9.5, regarded as critical for P release (Seitzinger, 1991), but values in late afternoon must have been markedly higher. Keto & Sammalkorpi (1988) noted a high pH gradient between the pelagial (9.5–9.7) and the littoral (10–10.5, sometimes even > 11) zones. However, due to typically lower pH in the sediment (Huser et al., 2016) which has a higher buffer capacity, the desorption effect of high pH in shallow area sediments may require resuspension of particles. In addition, Koski-Vähälä et al. (2001) demonstrated that in the Enonselkä basin the high silicon concentration strongly increases P concentration in the interstitial water of the oxic sediment. Interestingly, in 1985 the accumulation of a small 6–7 µm diameter Stephanodiscus diatom, often dominant in the Enonselkä basin in spring, was at its highest since the waste-water diversion (Liukkonen et al., 1993), which may have amplified the dissolution of silicon in the sediment. Thus, temporarily increased leaching of P from both the shallow and deepwater sediment plausibly created a spiral whereby nutrient leakages from anoxic and oxic sediment were sustaining cyanobacteria blooms and increasing oxygen consumption in the hypolimnion. Hence, although anoxia in the Enonselkä basin has generally been the consequence rather than the cause of elevated nutrient concentrations and sediment has been a sink of nutrients rather than their source, temporary reversals have been possible under suitable conditions.
The importance of nutrients leaching from the deepwater sediment is difficult to evaluate, because the vertical sampling interval is generally too scarce to cope with the steep concentration gradient in the neighbourhood of the sediment. In the Enonselkä basin, the depths deeper than 25 m represent only ~ 1% of the total water volume, so that extremely high concentrations from deep water are needed to cause a significant increase in the nutrient concentration of the whole water column. The extrapolation of the detailed vertical results in winter 1990 to the year 1986, when TP concentration at 29 m depth was over an order of magnitude higher, suggests that, exceptionally, nutrient concentrations of the whole water mass can be substantially elevated. The paradoxical alternation of low and high concentrations of deepwater nutrients in winter in different years is another weak point particularly from the management perspective. The most likely explanation for the paradox may be the variation in water and sediment temperatures at the time of lake freezing. Rather small temperature differences may create widely contrasting under-ice flow regimes (Salonen et al., 2014), which can explain dramatic differences between successive years. If necessary, consequences unfavourable conditions could be avoided by oxygenation, but the prediction of such cases may be challenging.
Development of phytoplankton
Because the Enonselkä basin feeds ~ 20% of the total inflow into the Kajaanselkä basin and its nutrient concentrations were roughly two times higher in late summer, it is reasonable that TP, TN and chlorophyll concentrations as well as Secchi depth in the Kajaanselkä basin roughly followed those of the Enonselkä basin (Fig. 8a–d). The positive anomalies of nutrient and chlorophyll concentrations in the respective dilution curves in the late 1980s were linked to the extremely high internal loading of nutrients in deep water at the same time in winter (Fig. 12c, d). However, no respective anomalies were found in the Kajaanselkä basin. The reduced relative variation in chlorophyll concentration in relation to its trend line between 1988 and 1997 (Fig. 8c) may have been due to grazers controlling the development of high phytoplankton densities in the Enonselkä basin. The low TN:TP ratio in deep water (Fig. 9b, d) in the last half of the 1980s may have favoured N fixing cyanobacteria which were dominant in the phytoplankton of the following summers. The absence of phytoplankton responses after respective bursts of nutrients in later winters can be explained by further advanced dilution of the waste water, a decreasing amount of nutrients leaching from the sediment (Jilbert et al., this issue), a decreasing external loading, and by a lack of weather-related factors favouring the internal release of nutrients in successive years.
In agreement with the decrease in phytoplankton biomass based on microscopic counting (Keto et al., 2005), about half of the decrease in the concentration of chlorophyll after its maximum in the late 1980s happened before the beginning of the intensive fish removal. Thus, the roughly 90% decrease in the biomass of cyanobacteria between 1988 and 1990 (Horppila et al., 1998) was likely more due to the decreasing availability of nutrients than to the biomanipulation. Instead, the decrease of chlorophyll concentration that was clearly below the level predicted by the dilution curve a few years after the intensive fish removal might be mainly attributed to the biomanipulation. This interpretation is corroborated by the absence of respective negative anomalies in nutrient concentrations in relation to their dilution curves (Fig. 8a, b) as well as the absence of a concomitant minimum in chlorophyll concentration in the Kajaanselkä basin around 1997. The ratio between Secchi depths of both basins (Fig. 8e) also indicates relative improvement of the Enonselkä basin in relation to the Kajaanselkä basin, while that was not the case in the respective ratio of TP results. No significant change in the chlorophyll concentration of Lake Pääjärvi between 1988 and 1997 (Fig. 11) suggests no regional trend that might affect the interpretation of the results of Lake Vesijärvi. The stabilization of chlorophyll concentration of the Enonselkä basin from 2000 corresponds to the experiences of other lakes in which a return to a more turbid state typically happens within 10 years or less after biomanipulation (Søndergaard et al., 2007). The virtual disappearance of the difference between the chlorophyll concentrations in early and late summer from the early 1990s suggests that the water quality of the Enonselkä basin had permanently reached a moderate condition in the ecological classification of the European Union’s Water Framework Directive.
Effect of fish removal
The effects of the 5-year intensive fish removal of the Enonselkä basin were gradually manifested in the decrease of fishable new recruits of planktivorous and benthivorous fish. The exceptionally strong year class of roach in 1988, which appeared from the littoral to the open water area a few years later (Horppila et al., 1998), could be removed by trawling. Although fish are able to move between the basins, the intensive fish removal predominantly depleted pelagic fish of the Enonselkä basin (Peltonen et al., 1999a, b). Fish removal meant a 90% decrease in planktivorous roach and smelt and after the collapse of the roach stock, the pelagic fish assemblage became smelt dominated with high year-to-year density variations (Ruuhijärvi et al., 2005). Similar to experiences elsewhere (e.g. Sarvala et al. 1998, 2001), the change led to a reduction in chlorophyll concentration by several parallel and/or successive mechanisms during and after the biomanipulation. The most direct effect was the removal of nutrients in fish biomass. According to Boros et al. (2012) the mean proportion of P in the dry mass of roach from six European lakes was 2.4%, which quite closely agrees with the results from four Polish lakes (Penczak et al., 1985) and Finnish Lake Köyliönjärvi (Tarvainen et al., 2002). This implies (assuming that dry mass is 25% of wet mass) that in 1989–1993 an average of 0.007 g P m−2 year−1 was removed from the Enonselkä basin, amounting to roughly 60% of the respective decrease in the concentration due to dilution and 12% of the estimated TP load. Thus, nutrients removed with fish no doubt advanced the recovery of the Enonselkä basin. Removal of nutrients during a short time at the end of a marked dilution phase of waste water from the mid-1970s also explains the stepwise decrease in nutrient concentrations to a rather stable level in 1993.
The top-down regulation of aquatic food chains suggested by Shapiro et al. (1975) is a widely accepted possibility but is influenced by a complex range of factors including species composition and size distributions of fish, zooplankton and phytoplankton (Ramcharan et al., 1996), which are further affected by the plastic behaviour of the organisms as well as stochastic weather conditions. Attention has often been placed on the role of large Daphnia species which are efficient filter feeders able to control phytoplankton but vulnerable to predation by fish (Brooks & Dodson, 1965). Because the relative P content of large Daphnia species is high compared with other zooplankton (e.g. Hessen & Andersen, 1992), the amount of P bound in their biomass can have fundamental ecological effects (e.g. Elser et al., 1996). However, in the Enonselkä basin large Daphnia species have not been prevalent and the increase in their mean carapace length was < 5% after the intensive fish removal (Nykänen et al., 2010). Anttila et al. (2013) interpreted the decrease in chlorophyll concentration of the Enonselkä basin during the biomanipulation as a major regime shift which included two stages, one in 1990 and another in 1993. The results of the present study support only a change in 1993 (Fig. 9) and even then the evidence that cladocerans were responsible hardly can be inferred only from the small increase in the size of Daphnia. In general, the idea of regime shift is controversial, because the alleged mechanistic links between pressures and ecological changes are often insufficiently demonstrated (Capon et al., 2015). In the case of the Enonselkä basin, the detection of possible transition points is further hampered by the simultaneous dilution of the waste water and the internal nutrient pulse in the late 1980s as well as the decreases in atmospheric and fertilizer loadings. Thus, as also concluded by Horppila et al. (1998), the change in the phytoplankton was not due to enhanced zooplankton grazing. There are also other examples that improvements in the water quality of lakes dominated by planktivorous fish that do not necessarily require significant changes in cladoceran populations (Hamrin, 1993; Sommer et al., 2001; Urrutia-Cordero et al., 2015) and top-down control of phytoplankton can be mediated by smaller cladocerans (Helminen & Sarvala, 1997).
The effect of the fish removal on phytoplankton of the Enonselkä basin was probably amplified by the stocking of over a million juvenile pikeperch (Sander lucioperca (L.)) in 1984–1991, which led to their reproduction in 1992 and to the establishment of a strong population supporting fisheries (Kairesalo et al., 1999). Compared with the 1980s, when open water predatory fish were scarce (Keto & Sammalkorpi, 1988), the change was remarkable and in this respect pikeperch stocking can be regarded as one of the greatest successes of the biomanipulation of the Enonselkä basin. The restitution of pikeperch seems to have created a new stable situation where the role of roach may have permanently declined. As an efficient open water predator chasing both smelt and roach (Peltonen et al., 1996), pikeperch probably became a deterrent keeping roach predominantly out of the pelagic zone, which was expressed as an increased size of individuals caught from the open water zone. Similar behaviour of roach has been found after pikeperch introduction in Lake Gjersjøen in southern Norway (Brabrand & Faafeng, 1993). Romare & Hansson (2003) and Gliwicz (2002) have also shown that the risk of predation can regulate how roach are distributed between the littoral and pelagial zones. Changes in the behaviour of fish and zooplankton emphasize the importance of sophisticated behavioural adaptations on top of simple mechanistic trophic relationships and render differentiation and quantification of various factors involved in biomanipulation extremely challenging.
General discussion
Although the basic mechanisms involved in lake ecosystems are universal, unique environmental conditions may markedly differ so that caution is needed particularly when comparing the results of small and large lakes or shallow and deep ones which have contrasting hydrodynamics. In lakes, such as Lake Vesijärvi, which are open to wind, stochastic hydrodynamic episodes can modify the food webs with annual or even longer timescale effects. The ostensible reversal of the favourable development of the Enonselkä basin 10 years after the waste-water diversion is a striking example. Then, as indicated by similar increases in nutrient and chlorophyll concentrations between the diversion of the sewage waters and the end of the 1980s (Fig. 8a–c), the phytoplankton in the Enonselkä basin was largely controlled by bottom-up factors. Instead, in later years, the low chlorophyll:TP ratio suggested a shift to the dominance of top-down factors. The complex interactions of planktivorous and benthivorous fish as well as macrophytes constitute a network of direct and indirect linkages between environmental and biological factors as well as between organisms at different trophic levels that also vary with time and space (Kairesalo et al., 1999). In agreement with Andersson et al. (1988) and Horppila & Kairesalo (1992) showed experimentally that benthivorous fish may radically increase nutrient flux from the sediment of the Enonselkä basin. Aquatic macrophytes may also affect phytoplankton by their nutrient competition (Hansson et al., 1998), but those were not included in regular monitoring of the Enonselkä basin. Increased water transparency in the middle of the 1990s allowed macrophytes to expand their habitat from ~ 2 m depth to ~ 4 m depth (Venetvaara & Lammi, 1995) so that finally they covered an area with roughly 50% of the water volume. Because the spreading of macrophytes into a new habitat and the growth of biomass takes time, their effects also appear with a delay (Scheffer et al., 1993). Therefore, the sudden drop in epilimnetic TP and TN concentrations between 1992 and 1993 (Fig. 9) was most likely mediated by the removal of benthivorous fish rather than by macrophytes.
Conclusions
The efforts to improve the water quality of the Enonselkä basin of Lake Vesijärvi from 1975 led to the re-establishment of sustainable fisheries and the recreational value of the lake. Although the progress was predominantly due to the diversion of the waste water and the following dilution, planktivorous and benthivorous fish removal and the stocking of predatory fish also supported the recovery. The detailed mechanisms remain unclear, but owing to the long-term monitoring and diverse approaches including comparison between the Enonselkä and Kajaanselkä basins, the main trend lines of the effects of the remediation measures could be elicited out of the multitude of interlinked factors mixed with stochastic weather conditions. The recovery of the Enonselkä basin has evidently reached a stable phase where the sediment and external load are the key factors determining the future recovery trajectory. According to the present results, the low-level fish removal and hypolimnetic oxygenation do not have great promise for the further improvement of water quality.
References
Andersen, J. M., 1975. Influence of pH on release of phosphorus from lake sediments. Archiv für Hydrobiologie 76: 411–419.
Andersen, J. M., 1982. Effect of nitrate concentration in lake water on phosphate release from the sediment. Water Research 16: 1119–1126.
Andersson, G., W. Granéli & J. Stenson, 1988. The influence of animals on phosphorus cycling in lake ecosystems. Hydrobiologia 170: 267–284.
Anttila, S., M. Ketola, K. Kuoppamäki & T. Kairesalo, 2013. Identification of a biomanipulation-driven regime shift in Lake Vesijärvi: implications for lake management. Freshwater Biology 58: 1494–1502.
Bendtsen, J., K. E. Gustafsson, J. Lehtoranta, E. Saarijärvi, K. Rasmus & H. Pitkänen, 2013. Modeling and tracer release experiment on forced buoyant plume convection from coastal oxygenation. Boreal Environment Research 18: 37–52.
Bernes, C., S. R. Carpenter, A. Gårdmark, P. Larsson, L. Persson, C. Skov, J. D. M. Speed & E. Van Donk, 2015. What is the influence of a reduction of planktivorous and benthivorous fish on water quality in temperate eutrophic lakes? A systematic review. Environmental Evidence 4: 1–28.
Beutel, M. W., 2006. Inhibition of ammonia release from anoxic profundal sediments in lakes using hypolimnetic oxygenation. Ecological Engineering 28: 271–279.
Beutel, M. W. & A. J. Horne, 1999. A review of the effects of hypolimnetic oxygenation on lake and reservoir water quality. Lake and Reservoir Management 15: 285–297.
Bormans, M., B. Maršálek & D. Jančula, 2016. Controlling internal phosphorus loading in lakes by physical methods to reduce cyanobacterial blooms: a review. Aquatic Ecology 50: 407–422.
Boros, G., J. Jyväsjärvi, P. Takács, A. Mozsár, I. Tátrai, M. Søndergaard & R. I. Jones, 2012. Between-lake variation in the elemental composition of roach (Rutilus rutilus L.). Aquatic Ecology 46: 385–394.
Brabrand, Å. & B. Faafeng, 1993. Habitat shift in roach (Rutilus rutilus) induced by pikeperch (Stizostedion lucioperca) introduction: predation risk versus pelagic behaviour. Oecologia 95: 38–46.
Brooks, J. L. & S. I. Dodson, 1965. Predation, body size, and composition of plankton. Science 150: 28–35.
Bryant, L. D., P. A. Gantzer & J. C. Little, 2011. Increased sediment oxygen uptake caused by oxygenation-induced hypolimnetic mixing. Water Research 45: 3692–3703.
Capon, S. J., A. J. J. Lynch, N. Bond, B. C. Chessman, J. Davis, N. Davidson, M. Finlayson, P. A. Gell, D. Hohnberg, C. Humphrey, R. T. Kingsford, D. Nielsen, J. R. Thomson, K. Ward & R. Mac Nally, 2015. Regime shifts, thresholds and multiple stable states in freshwater ecosystems; a critical appraisal of the evidence. Science of the Total Environment 534: 122–130.
Carpenter, S. R., 2005. Eutrophication of aquatic ecosystems: bistability and soil phosphorus. Proceedings of the National Academy of Sciences of the United States of America 102: 1003–1005.
Drenner, R. W. & K. D. Hambright, 2002. Piscivores, trophic cascades, and lake management. The Scientific World Journal 2: 284–307.
Einsele, W., 1936. Über die Beziehungen des Eisenkreislaufs zum Phosphatkreislauf im eutrophen See. Archiv für Hydrobiologie 29: 664–686.
Elser, J. J., D. R. Dobberfuhl, N. A. MacKay & J. H. Schampel, 1996. Organism size, life history, and N: P stoichiometry, toward a unified view of cellular and ecosystem processes. BioScience 46: 674–684.
Engstrom, D. R. & D. I. Wright, 2002. Sedimentological effects of aeration-induced lake circulation. Lake and Reservoir Management 18: 201–214.
Gliwicz, Z. M., 2002. On the different nature of top-down and bottom-up effects in pelagic food webs. Freshwater Biology 47: 2296–2312.
Gächter, R. & B. Wehrli, 1998. Ten years of artificial mixing and oxygenation: no effect on the internal phosphorus loading of two eutrophic lakes. Environmental Science and Technology 32: 3659–3665.
Hansson, L.-A., H. Annadotter, E. Bergman, S. F. Hamrin, E. Jeppesen, T. Kairesalo, E. Luokkanen, P.-A. Nilsson, M. Søndergaard & J. Strand, 1998. Biomanipulation as an application of food-chain theory: constraints, synthesis, and recommendations for temperate lakes. Ecosystems 1: 558–574.
Hamrin, S., 1993. Lake restoration by cyprinid control in Sätofta Bay (Lake Ringsjön). Verhandlungen der internationale Vereinigung für theoretische und angewandte Limnologie 25: 487–493.
Helminen, H. & J. Sarvala, 1997. Responses of Lake Pyhäjärvi (SW Finland) to variable recruitment of the major planktivorous fish, vendace (Coregonus albula). Canadian Journal of Fisheries and Aquatic Sciences 54: 32–40.
Hessen, D. O. & T. Andersen, 1992. The algae-grazer interface: feedback mechanisms linked to elemental ratios and nutrient cycling. Ergebnisse der Limnologie 35: 111–120.
Holmroos, H., J. Horppila, S. Laakso, J. Niemistö & S. Hietanen, 2016. Aeration-induced changes in temperature and nitrogen dynamics in a dimictic lake. Journal of Environmental Quality 45: 1359–1366.
Horppila, J. & T. Kairesalo, 1992. Impacts of bleak (Alburnus alburnus) and roach (Rutilus rutilus) on water quality, sedimentation and internal nutrient loading. Hydrobiologia 243(244): 323–333.
Horppila, J., H. Peltonen, T. Malinen, E. Luokkanen & T. Kairesalo, 1998. Top-down or bottom-up effects by fish: issues of concern in biomanipulation of lakes. Restoration Ecology 6: 20–28.
Hupfer, M. & J. Lewandowski, 2008. Oxygen controls phosphorus release from lake sediments – a long lasting paradigm in limnology. International Review of Hydrobiology 93: 415–432.
Huser, B. J., S. Egemose, H. Harper, M. Hupfer, H. Jensen, K. M. Pilgrim, K. Reitzel, E. Rydin & M. Futter, 2016. Longevity and effectiveness of aluminium addition to reduce sediment phosphorus release and restore lake water quality. Water Research 97: 122–132.
Jeppesen, E., M. Søndergaard, J. P. Jensen, K. Havens, O. Anneville, L. Carvalho, M. F. Coveney, R. Deneke, M. Dokulil, B. Foy, D. Gerdeaux, S. E. Hampton, K. Kangur, J. Köhler, S. Körner, E. Lammens, T. L. Lauridsen, M. Manca, R. Miracle, B. Moss, P. Nõges, G. Persson, G. Phillips, R. Portielje, S. Romo, C. L. Schelske, D. Straile, I. Tatrai, E. Willén & M. Winder, 2005. Lake responses to reduced nutrient loading – an analysis of contemporary long-term data from 35 case studies. Freshwater Biology 50: 1747–1771.
Jilbert, T., S. Jokinen, T. Saarinen, U. Mattus-Kumpunen, A. Simojoki, S. Saarni, S. Salminen, J. Niemistö & J Horppila, 2020. Impacts of a deep reactive layer on sedimentary phosphorus dynamics in a boreal lake recovering from eutrophication. Hydrobiologia.
Kairesalo, T., S. Laine, E. Luokkanen, T. Malinen & J. Keto, 1999. Direct and indirect mechanisms behind successful biomanipulation. Hydrobiologia 395(396): 99–106.
Keto, J., 1982. The recovery of Lake Vesijärvi after sewage diversion. Hydrobiologia 86: 195–199.
Keto, J. & I. Sammalkorpi, 1988. A fading recovery: a conceptual model for Lake Vesijärvi management and research. Aqua Fennica 18: 193–204.
Keto, J. & P. Tallberg, 2000. The recovery of Vesijärvi, a lake in southern Finland: water quality and phytoplankton interpretations. Boreal Environment Research 5: 15–26.
Keto, J., P. Tallberg, I. Malin, P. Vääränen & K. Vakkilainen, 2005. The horizon of hope for L. Vesijärvi, southern Finland: 30 years of water quality and phytoplankton studies. Verhandlungen der internationale Vereinigung für theoretische und angewandte Limnologie 29: 448–452.
Koski-Vähälä, J., H. Hartikainen & P. Tallberg, 2001. Phosphorus mobilization from various sediment pools in response to increased pH and silicate concentration. Journal of Environmental Quality 30: 546–552.
Kuha, J. K., A. H. Palomäki, J. T. Keskinen & J. S. Karjalainen, 2016. Negligible effect of hypolimnetic oxygenation on the trophic state of Lake Jyväsjärvi, Finland. Limnologica 58: 1–6.
Kuusisto E., 2012. Hydrology of Lake Vesijärvi. In: Keto, J., H. Kolunen, A. Pekkarinen & L. Tuominen (eds), Lake Vesijärvi daughter of the Salpausselkä Ridges: 59–61.
Lappalainen, K. M., 1994. Positive changes in oxygen and nutrient contents in two Finnish lakes induced by Mixox hypolimnetic oxygenation method. Verhandlungen der internationale Vereinigung für theoretische und angewandte Limnologie 25: 2510–2513.
Lee, G. F., W. C. Sonzogni & R. D. Spear, 1977. Significance of oxic versus anoxic conditions for lake Mendota sediment phosphorus release. Proceedings of the International Symposium on Interactions Between Sediments and Fresh Water, Amsterdam, W. Junk, The Hague: 294–306.
Liboriussen, L., M. Søndergaard, E. Jeppesen, I. Thorsgaard, S. Grünfeld, T. S. Jakobsen & K. Hansen, 2009. Effects of hypolimnetic oxygenation on water quality: results from five Danish lakes. Hydrobiologia 625: 157–172.
Liukkonen, M., T. Kairesalo & J. Keto, 1993. Eutrophication and recovery of Lake Vesijärvi (south Finland): Diatom frustules in varved sediments over a 30-year period. Hydrobiologia 269(270): 415–426.
Mazumder, A., 1994. Phosphorus-chlorophyll relationships under contrasting herbivory and thermal stratification: predictions and patterns. Canadian Journal of Fisheries and Aquatic Sciences 51: 390–400.
MacIntyre, S., K. M. Flynn, R. Jellison & J. R. Romero, 1999. Boundary mixing and nutrient flux in Mono Lake, California. Limnology and Oceanography 44: 512–529.
Mehner, T., 2010. No empirical evidence for community-wide top-down control of prey fish density and size by fish predators in lakes. Limnology and Oceanography 55: 203–213.
Mortimer, C. H., 1941. The exchange of dissolved substances between mud and water in lakes. Journal of Ecology 29: 280–329.
Niemistö, J., S. Silvonen & J. Horppila, 2020. Effects of hypolimnetic aeration on the quantity and quality of settling material in a eutrophied dimictic lake. Hydrobiologia. https://doi.org/10.1007/s10750-019-04160-6.
Nürnberg, G. K., 2009. Assessing internal phosphorus load – problems to be solved. Lake and Reservoir Management 25: 419–432.
Nygrén, N. A., P. Tapio & J. Horppila, 2017. Will the oxygen-phosphorus paradigm persist? – expert views of the future of management and restoration of eutrophic lakes. Environmental Management 60: 947–960.
Nykänen, M., T. Malinen, K. Vakkilainen, M. Liukkonen & T. Kairesalo, 2010. Cladoceran community responses to biomanipulation and re-oligotrophication in Lake Vesijärvi, Finland, as inferred from remains in annually laminated sediment. Freshwater Biology 55: 1164–1181.
Ohle, W., 1978. Ebullition of gases from sediment, condition, and relationship to primary production of lakes. Verhandlungen der internationale Vereinigung für theoretische und angewandte Limnologie 20: 957–962.
Peltonen, H., H. Rita & J. Ruuhijärvi, 1996. Diet and prey selection of pikeperch (Stizostedion lucioperca (L.)) in Lake Vesijärvi analysed with a logit model. Annales Zoologici Fennici 33: 481–487.
Peltonen, H., J. Ruuhijärvi, T. Malinen, J. Horppila, M. Olin & J. Keto, 1999a. The effects of food-web management on fish assemblage dynamics in a north temperate lake. Journal of Fish Biology 55: 54–67.
Peltonen, H., J. Ruuhijärvi, T. Malinen & J. Horppila, 1999b. Estimation of roach (Rutilus rutilus (L.)) and smelt (Osmerus eperlanus (L.)) stocks with virtual population analysis, hydroacoustics and gillnet CPUE. Fisheries Research 44: 25–36.
Penczak, T., M. Moliński, W. Galicka & A. Prejs, 1985. Factors affecting nutrient budget in lakes of R. Jorka watershed (Masurian Lakeland, Poland) VII. Input and removal of nutrients with fish. Ekologia Polska 33: 301–309.
Preece, E. P., B. C. Moore, M. M. Skinner, A. Child & S. Dent, 2019. A review of the biological and chemical effects of hypolimnetic oxygenation. Lake and Reservoir Management 35: 229–246.
Ramcharan, C. W., R. L. France & D. J. McQueen, 1996. Multiple effects of planktivorous fish on algae through a pelagic trophic cascade. Canadian Journal of Fisheries and Aquatic Sciences 53: 2819–2827.
Romare, P. & L. A. Hansson, 2003. A behavioral cascade: top predator induced behavioral shifts in planktivorous fish and zooplankton. Limnology and Oceanography 48: 1956–1964.
Ruuhijärvi, J., T. Malinen, P. Ala-Opas & A. Tuomaala, 2005. Fish stocks of Lake Vesijärvi: from nuisance to flourishing fishery in 15 years. Verhandlungen der internationale Vereinigung für theoretische und angewandte Limnologie 29: 384–389.
Ruuhijärvi, J., T. Malinen, K. Kuoppamäki, P. Ala-Opas & M. Vinni, 2020. Responses of food web to artificial circulation in Lake Vesijärvi. Hydrobiologia (this issue).
Salmi, P., I. Malin & K. Salonen, 2014. Pumping of epilimnetic water into hypolimnion improves oxygen but not necessarily nutrient conditions in a lake recovering from eutrophication. Inland Waters 4: 425–434.
Salonen, K., M. Pulkkanen, P. Salmi & R. W. Griffiths, 2014. Interannual variability of circulation under spring ice in a boreal lake. Limnology and Oceanography 59: 2121–2132.
Sarvala, J., H. Helminen, V. Saarikari, S. Salonen & K. Vuorio, 1998. Relations between planktivorous fish abundance, zooplankton and phytoplankton in three lakes of differing productivity. Hydrobiologia 363: 81–95.
Sarvala, J., H. Helminen & J. Karjalainen, 2001. Restoration of Finnish lakes using fish removal: changes in the chlorophyll-phosphorus relationship indicate multiple controlling mechanisms. Verhandlungen der Internationale Vereinigung für theoretische und angewandte Limnologie 27: 1473–1479.
Sarvala, J., H. Helminen & J. Heikkilä, 2020. Invasive submerged macrophytes complicate management of a shallow boreal lake: a 42-year history of monitoring and restoration attempts in Littoistenja¨rvi, SW Finland. Hydrobiologia. https://doi.org/10.1007/s10750-020-04318-7.
Scheffer, M., S. H. Hosper, M.-L. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology & Evolution 8: 275–279.
Seitzinger, S. P., 1991. The effect of pH on the release of phosphorus from Potomac Estuary sediments: implications for blue-green algal blooms. Estuarine Coastal and Shelf Science 33: 409–418.
Shapiro, J., V. Lamarra & M. Lynch, 1975. Biomanipulation: an ecosystem approach to lake restoration. In Brezonik, P. L. & J. F. Fox (eds), Water Quality Management Through Biological Control. Univ. of Florida, Gainesvill: 85–96.
Sommer, U., F. Sommer, B. Santer, C. Jamieson, M. Boersma, C. Becker & T. Hansen, 2001. Complementary impact of copepods and cladocerans on phytoplankton. Ecology Letters 4: 545–550.
Søndergaard, M., P. Kristensen & E. Jeppesen, 1993. Eight years of internal phosphorus loading and changes in the sediment phosphorus profile of Lake Søbygaard, Denmark. Hydrobiologia 253: 345–356.
Søndergaard, M., E. Jeppesen, T. L. Lauridsen, C. Skov, E. H. Van Nes, R. Roijackers, E. Lammens & R. Portielje, 2007. Lake restoration: successes, failures and long-term effects. Journal of Applied Ecology 44: 1095–1105.
Tammeorg, O., T. Möls, J. Niemistö, H. Holmroos & J. Horppila, 2017. The actual role of oxygen deficit in the linkage of the water quality and benthic phosphorus release: potential implications for lake restoration. Science of the Total Environment 599–600: 732–738.
Tarvainen, M., J. Sarvala & H. Helminen, 2002. The role of phosphorus release by roach (Rutilus rutilus) in the water quality changes of a biomanipulated lake. Freshwater Biology 47: 2325–2336.
Urrutia-Cordero, P., M. K. Ekvall & L.-A. Hansson, 2015. Responses of cyanobacteria to herbivorous zooplankton across predator regimes: who mows the bloom? Freshwater Biology 60: 960–972.
Venetvaara, J. & E. Lammi, 1995. Vesijärven kasvillisuuden nykytila ja viimeaikaiset muutokset (in Finnish with English abstract). In Sammalkorpi, I., J. Keto, T. Kairesalo, E. Luokkanen, M. Mäkelä, J. Vääriskoski & E. Lammi (Eds), Lake Vesijärvi Project: Mass Removal of Cyprinids and Traditional Water Protection. Publications of Water and Environment Administration – Series A 218: 101–106.
Acknowledgements
Open access funding provided by University of Helsinki including Helsinki University Central Hospital. This study is a product of the long-term efforts of many parties. To mention only the main players, the city of Lahti played a pivotal role in initiating and running the monitoring programme as well as providing an attractive environment for various research projects. Numerous persons in many organizations have contributed to the production of data over the years. The establishment of the Vesijärvi Foundation greatly strengthened the Lake Vesijärvi research. We thank the anonymous reviewers and Tom Jilbert for their constructive comments. Cathryn Primrose-Mathisen kindly checked the English language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Tom Jilbert, Raoul-Marie Couture, Brian J. Huser & Kalevi Salonen / Restoration of eutrophic lakes: current practices and future challenges
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salonen, K., Sarvala, J., Horppila, J. et al. Development of Lake Vesijärvi through four decades of remediation efforts. Hydrobiologia 847, 4601–4619 (2020). https://doi.org/10.1007/s10750-020-04338-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04338-3