Abstract
Wastewaters discharged to Lake Vesijärvi ruined its recreational value and demanded mitigation measures. In the mid-1970s, the diversion of wastewaters elsewhere reduced epilimnetic total phosphorus concentration by ~ 40% from > 100 mg P m−3 in 5 years, but this was not enough to eliminate cyanobacterial blooms. In 1979–1984, hypolimnetic oxygenation was applied to decrease internal nutrient loading, but pumping warm surface water to the hypolimnion probably intensified gas ebullition from the sediment, carrying nutrients to the epilimnion and intensifying cyanobacterial blooms. Intensive fish removal in 1989–1994 was more successful. Five years of summer trawling removed over three-quarters of roach and smelt stock, and after the two most intensive fishing years, TP and chlorophyll concentrations abruptly decreased by ~ 35%. During subsequent years fish removal continued at ~ 30% intensity and maintained chlorophyll and total nutrient concentrations at a lower level until the end of the study. At the same time, the frequency of cyanobacterial blooms decreased from annual to twice per decade. Larger-scale oxygenation after 2010 no longer resulted in ebullition, and its effect on phytoplankton was indistinguishable from natural variability. Consequently, it was abandoned. The intensity of fish removal needed to maintain the present status of the lake is still awaiting evaluation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eutrophication of lakes is a global problem that culminates in high phytoplankton biomass, often cyanobacteria. Intense cyanobacterial blooms typically dominate in a particular stage of eutrophication (Jeppesen et al., 2005), lowering the recreational value of the lake and causing aesthetic disadvantages as well as potential health risks (e.g., Svirčev et al., 2019).
Because eutrophication is typical in lakes near dense human populations, it has received wide attention, and counteractive measures have been developed. However, the consequences of eutrophication have proven to be slow to overcome. The most challenging problem is the leaching of legacy nutrients from the sediment, which may take decades to balance (Søndergaard et al., 2007) after the reduction of external loading. It is seldom possible to eliminate the internal loading of legacy nutrients from the sediment by clay coverage, dredging, hypolimnetic water removal, etc. Therefore, in situations in which the external loading of nutrients cannot be sufficiently reduced, other means have been developed to mitigate the problems caused by high phytoplankton biomass and cyanobacterial blooms. Unfortunately, it is not a straightforward one-step measure but requires ongoing or periodic investments and monitoring.
Every lake has a specific combination of features and therefore approaches to restoration need to be site-specific rather than universal. Furthermore, stochastic weather dynamics produce a broad spectrum of hydrological conditions and highly variable hydrodynamics. Because so many, often stochastic, factors affect the complicated trophic interactions within pelagic and benthic food webs, the biocoenoses of successive years are hardly ever similar, irrespective of seemingly identical circumstances. Therefore, in remediation studies, the response time of lakes to remediation measures is variable (Chorus et al., 2020); short-term changes may not reflect the real rate of change in water quality.
Recovery of a lake from eutrophication is a holistic ecosystem process, whose management requires data for numerous parameters. Further, long time series and experimental approaches are needed to understand the mechanisms behind successes or failures. However, costs for studies over many years can be substantial, requiring long-term commitment. Consequently, long time series are rare. Food web interactions modified by weather-related drivers make studying tiny and rapidly growing organisms, such as phytoplankton, particularly challenging. Because phytoplankton strongly affects a lake's recreational and economic value, it is directly or indirectly often the main target of remediation measures and a necessary parameter for monitoring the state of the lake (Carvalho et al., 2012).
Lake Vesijärvi received wastewaters from the City of Lahti for decades, which led to severe eutrophication where high phytoplankton biomass and harmful cyanobacterial blooms destroyed its fisheries and recreational value. These problems forced administrators to start a series of measures (Keto & Sammalkorpi, 1988) to reduce external nutrient loading and to improve water quality by other means. Many approaches were applied successively and in parallel, but the main ones were oxygenation and biomanipulation.
A variety of oxygenation methods aim to limit the decrease in redox potential to a level at which P release from the anoxic sediment no longer occurs (Bormans et al., 2016), and the flux of nutrients from the anoxic hypolimnion does not increase phytoplankton growth. However, redox-sensitive release of nutrients may not be the only key source of nutrients from the sediment, even when hypolimnion is anoxic, e.g. dissolution of calcium-bound P or decomposition of organic P may be more important (Gächter & Wehrli, 1998; Hupfer & Lewandowski, 2008; Salmi et al., 2014; Orihel et al., 2017). In particular, biological activities like the respiration of micro-organisms (Prairie et al., 2001) and perturbation of sediment by invertebrates (Chakraborty et al., 2022) are of significance.
The most common biomanipulation approaches aim to enhance zooplankton grazing on phytoplankton (Carpenter et al., 1985). Although manipulation of fish communities can be used to control phytoplankton, Brett & Goldman (1996) suggested that even aggressive biomanipulation of zooplanktivorous fish may sometimes only minimally reduce phytoplankton biomass. Particularly in small lakes, increasing the biomass of aquatic plants can be an efficient biomanipulation leading to an alternative state (Scheffer et al., 1993), where nutrient uptake by aquatic plants and periphyton limit phytoplankton biomass (Petr, 2000; Dokulil et al., 2018). In addition, fish removal targeting benthivorous fish can reduce sediment disturbance and the flux of nutrients from the sediment available for phytoplankton (e.g., Horppila et al., 1998).
The history of eutrophication and the effects of the main remediation measures on the physical and chemical conditions of Lake Vesijärvi have been earlier reported in detail by Horppila et al. (1998), Keto & Tallberg (2000), Keto et al. (2005), Salmi et al. (2014) and Salonen et al. (2020). In this study, we used more than 40 years of data to analyze how oxygenation and biomanipulation of Lake Vesijärvi affected phytoplankton species composition and biomass. We hypothesized that the new data from 27 years after the earlier evaluation of phytoplankton development in Lake Vesijärvi (Keto et al., 2005) provide a broader vision to interpret the results and enable the development of a more effective strategy for the future monitoring and control of cyanobacteria of the lake.
Materials and methods
Study site
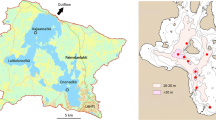
Lake Vesijärvi is located between the Salpausselkä ridges created by the last glaciation. Its southern basin, Enonselkä, is bordered by the City of Lahti, with about 120, 000 inhabitants. The 33 m deep Enonselkä basin (mean depth 6.1 m, area 32 km2) has a relatively long residence time, roughly 9 years. The present estimate is higher than before, mainly because the uptake of drinking water by the City of Lahti has diminished natural groundwater discharge into the lake. The wastewaters from the City of Lahti were discharged into the Enonselkä basin until the mid-1970s when they were diverted elsewhere, and the external load of ~ 2 g m−2 P year−1 was cut by order of magnitude (Keto & Sammalkorpi, 1988). In the 1980s, the diversion of industrial wastewaters further reduced the nutrient load by 17% (Horppila et al., 1998). The decrease in nutrient load also affected the Kajaanselkä basin (depth 42 m, mean depth 6.8 m, area 44 km2, residence time 2.4 years), which receives ~ 20% of the inflow from the Enonselkä basin. A more detailed description of the lake is given by Salonen et al. (2020).
Remediation methods
To increase deep-water oxygen concentration, a pump station was installed for winter operation in the deepest part of the Enonselkä basin in 1979. In 1983, the number of pumps was increased to three, and they were also operated in the summer until 1985. Water was pumped from 1 m depth to the hypolimnion 8–10 m above the sediment surface to avoid resuspension (Lappalainen, 1994). The same approach was applied extensively from 2010 to 2018 with eight 2.5 kW pump stations and one 1.5 kW station above the Enonselkä basin's deeps (more details in Salmi et al., 2014; Ruuhijärvi et al., 2020; Salonen et al., 2020).
Intensive removal of planktivorous and benthivorous fish (mainly roach and smelt) from the Enonselkä basin was performed by trawling over the area deeper than 10 m (in 1989) or 5–6 m (in 1990–1993) in May–August (Kairesalo et al., 1999; Keto & Tallberg, 2000). However, from 1994 until today, trawling was replaced by fyke net fishing in spring–summer and seine (when possible, also in winter) fishing in autumn. In recent years, fish traps were also used in winter.
Environmental conditions
To limit the effects of variable timing of vernal and autumnal overturn causing high random variation in nutrient concentrations and phytoplankton biomass, the main emphasis in lake data was put on the results of June–August. Total nitrogen (TN) and total phosphorus (TP) concentrations of 1 m depth, as well as Secchi depth at the deepest points of the Enonselkä and Kajaanselkä basins, were used as background data for the development of phytoplankton because the results of inorganic nutrients (NH4–N, NO3–N, and PO4–P) at low concentrations suffered from analytical challenges, high variation, and incomplete time series. Besides, as nutrient uptake is saturated above specific concentrations, it then has no effect on phytoplankton biomass.
The Finnish Meteorological Institute measured temperature and precipitation at the Lahti Laune (since 2019 Sopenkorpi) weather station. It also measured the atmospheric deposition of inorganic N at the Evo integrated monitoring site within a pristine forest area (~ 40 km distance from Lake Vesijärvi). We used statistics of fertilizers sold to farmers in Finland to estimate the development of the nutrient load to the lake from agriculture.
Phytoplankton
Water samples were taken in summer from the epilimnion of the deepest parts of the basins with a Ruttner or Limnos tube sampler, usually once a month. Phytoplankton was preserved with acid Lugol's solution and stored in darkness before counting. Settled (Utermöhl, 1958) phytoplankton was counted using an inverted microscope and phase-contrast or brightfield illumination with two or three magnifications (total magnifications 100×–1000×). Typically, at least 500 counting units (cells, colonies, or filaments) were counted in each sample. Wet biomasses were calculated using the list of the biovolumes for different species and their size classes, assuming that the density of phytoplankton is the same as that of water. Keto & Tallberg (2000) published more detailed taxon-specific data from 1982 to 1994.
Statistical analyses
The graphics, correlation, and regression analyses were made with Microsoft Excel 2016. Mann–Whitney U-tests and p-values for Pearson correlations were calculated using Quick Statistics Calculators (https://www.socscistatistics.com). A segmented linear regression model (SegReg free calculator for segmented piecewise regression breakpoint, waterlog.info/segreg.htm) was used to identify the breakpoints of chlorophyll concentration and phytoplankton biomass with varying TP concentration.
Results
Air temperature and precipitation
The mean air temperature in June–August (Fig. 1) increased by 0.023 ℃ year−1 during the study period (R2 = 0.15, P = 0.003), which increased the average length of the ice-free period in the Enonselkä basin by 1.05 d year−1 (1979–2020, R2 = 0.44, P < 0.001). While there was no significant trend (R2 = 0.06 in the annual precipitation between 1988 and 1997, the summer precipitation (from June to August) consistently decreased (R2 = 0.57, P = 0.01), for that period, and the interannual variation related to the trend was exceptionally low.
Hypolimnetic oxygen concentration
The late summer hypolimnetic oxygen concentration in the Enonselkä basin was highly variable (Fig. 2). After the diversion of the wastewaters of the City of Lahti, the concentration at the depth of 29 m varied between anoxic and hypoxic without any marked improvement until the large-scale oxygenation started in 2010. The most consistent period of low oxygen occurred in 1996–2010.
Hypolimnetic oxygen concentration at the end of summer stratification at 29 m depth in the Enonselkä basin and 39 m depth in the Kajaanselkä basin. The bar explanations are as in Fig. 1
Fish removal and introductions
The largest amount of fish was removed from the Enonselkä basin in 1991–1992 (Fig. 3), but in the following years until the end of the study, annual fish removal decreased on average to about 30% of that. Fish removal was complemented by annual introductions of, on average, 76,000 fingerlings year−1 (mean length 70 mm, CV 11%) of predatory pikeperch, Stizostedion lucioperca (L.). The highest introductions were during the intensive fish removal (average 146,000 ind. year−1 for 1989–1993). Unfortunately, after 2008 the statistics were no longer compiled by public authorities; therefore, the subsequent results may be underestimated. Initially, the introductions were to the Enonselkä basin, but later to all basins. However, due to fish mobility, probably all basins were affected.
Annual amount of phosphorus and nitrogen fertilizers sold to farmers (per field area) in Finland, atmospheric N deposition, number of introduced pikeperch, and removal of planktivorous and benthivorous fish in the Enonselkä basin. The bar explanations are as in Fig. 1
Nutrient load
The use of fertilizers in agriculture steadily increased (~ 40% for N and ~ 20% for P) in Finland between the mid-1970s and 1990 (Fig. 3), but later new recommendations led to rapid decreases in the amounts of fertilizers used. Between 1990 and 1997, the use of P and N fertilizers decreased on average by 8.8% year−1 and 3.3% year−1, respectively. Later the average decrease rates slowed to 2.4% year−1 and 0.7% year−1, respectively. The reduction in atmospheric N deposition was similarly high (6.5% year−1 and 2.1% year−1). Possible changes in nutrient loadings from natural or urban runoff could not be precisely estimated.
Epilimnetic nutrient concentration
After the diversion of the sewage waters, the epilimnetic concentrations of TN and TP in the Enonselkä basin (Fig. 4a, b) decreased from > 100 mg m−3 to ~ 50 mg m−3 TP and ~ 1500 mg m−3 to ~ 600 mg m−3 TN by the end of the 1970s before the end of the first oxygenation period (light green bar in Fig. 4). These new levels persisted until 1993, when the concentrations suddenly declined by 30–40% at the end of the efficient fishing period. The further decrease was slow and statistically significant only for TN (Table 1). Although oxygenation improved hypolimnetic oxygen concentration (Fig. 2) its effect was not detected in the epilimnetic nutrient concentrations (green bars in Fig. 4a, b). In the Kajaanselkä basin, high nutrient concentrations before the wastewater diversion from the Enonselkä basin were less distinct. Later, the developments of TP and TN concentrations closely corresponded to those of the Enonselkä basin, at a lower level (on average 59% and 78% of the respective concentration in the Enonselkä basin). Again, the decrease was statistically significant only for TN.
a-e Mean concentration of TP, TN, and chlorophyll as well as phytoplankton biomass and Secchi depth; f standardized [(value minus mean)/standard deviation] ratios between the results of the Enonselkä and Kajaanselkä basins Vertical bars – Range of the results; The bar explanations are as in Fig. 1
The mean (molar) TN:TP ratio in the epilimnion of the Enonselkä basin increased (R2 = 0.2113, P < 0.001) fairly steadily in June–August during the study period (Fig. 5). In the Kajaanselkä basin, variation of TN:TP ratio was high in the early phase of the study and the trend was not significant (R2 = 0.022, P = 0.31). However, after the 1980s the development closely followed that of the Enonselkä basin at about 20 units higher level. The similar decreases in both basins after 2005 (Fig. 4) suggest common reason(s).
a TN:TP ratio, b Coefficient of variation of intra-annual phytoplankton biomass and chlorophyll concentration, and c standardized chlorophyll:TP and phytoplankton biomass:TP ratio of the Enonselkä and Kajaanselkä basins. All lines represent five-year moving averages. Thick lines – Biomass; Dashed lines – Chlorophyll concentration; Thin dotted line – Linear regression. The bar explanations are as in Fig. 1
Secchi depth, chlorophyll, and phytoplankton biomass
Soon after the wastewater diversion, the Secchi depth of the Enonselkä basin increased from about 1 m to almost 2 m, but before the mid-1980s, it decreased to ~ 1.5 m (Fig. 4c). During the intensive fish removal, it started to increase again and reached a 2.9 m annual average in 1997, while later annual average varied around 2 m depth. However, sporadically high values also occurred, corresponding to those observed in 1994–1997. Furthermore, the Secchi depth values after 1993 were significantly higher (Mann–Whitney U-test, P = 0.035) than before, and their coefficient of variation was also lower (0.17 vs. earlier 0.23). During the large-scale oxygenation in 2010–2018, Secchi depth decreased to about 2 m. In the Kajaanselkä basin, Secchi depth followed a similar course (r = 0.44, P = 0.002), but the difference between the basins decreased from an average of 1 m in 1977–1992 to 0.7 m in 1993–2020. Because the changes were very similar, it is difficult to know how much Secchi depth of the Enonselkä basin was affected by intensive fish removal or oxygenation.
Chlorophyll concentration in the Enonselkä basin decreased rapidly after the wastewater diversion (Fig. 4d), but in the mid-1980s, it returned to a high level of ~ 25 mg m−3. Around 1987 a consistent decrease started, and within ten years, the concentration decreased to a third. At the same time, the coefficient of variation (CV) of annual results decreased from ~ 50% to ~ 30% (Fig. 5a), indicating more stable conditions. Between 1993 and 2020, the concentration increased slightly (r = 0.399 P = 0.02) and intra-annual variation increased. During the large-scale oxygenation (2010–2018), chlorophyll concentration was not significantly different from that during the ten preceding years (Mann–Whitney U-test). In the 1980s, concentration in the Enonselkä basin was roughly four times higher than in the Kajaanselkä basin, but in the early 1990s, the difference had halved. The significant correlation between the results of the two basins (r = 0.49, P < 0.01) and the similarity between the time courses of the relative variation of both chlorophyll concentration and phytoplankton biomass (Fig. 5b) suggest that the inflow from Enonselkä to Kajaanselkä and concurrent external drivers affected phytoplankton in the Kajaanselkä basin. The interannual variation of biomass (mean CV 56% in Enonselkä and 54% in Kajaanselkä) was significantly higher (Mann–Whitney U-test, P < 0.001) than that of chlorophyll concentration (mean CV 37% and 40%, respectively).
Chlorophyll:TP and phytoplankton biomass:TP ratios (Fig. 5c) reflect the proportion of P allocated in phytoplankton biomass, thus potentially indicating the food web structure. Their time courses related to the other factors, and the coherence between the basins corroborate that the observed trends are related to common factors. In the Enonselkä basin, both ratios decreased during the intensive fish removal (brown bar, Fig. 5) until the mid-1990s, but after 2000, they increased again. Chlorophyll:TP ratio of the Kajaanselkä basin correlated significantly with that of the Enonselkä basin (r = 0.33, P = 0.03), but the respective correlation for phytoplankton biomass:TP, based on fewer samples, was insignificant (r = 0.21, P = 0.22).
Development of chlorophyll and phytoplankton biomass related to TP showed turning points (Fig. 6) whose timings match the most intensive fish removal years between 1989 and 1993. In the Kajaaselkä basin points were faintly evident. Chlorophyll concentration in the Enonselkä basin decreased quite linearly with TP concentration (R2 = 0.80, P < 0.001) (Fig. 6), but due to cyanobacteria, the relationship with phytoplankton biomass was nonlinear. Without cyanobacteria, phytoplankton biomasses were not significantly different (mainly due to the results of 1985) before and after 1993 (Mann–Whitney U-test, P = 0.15). In the Kajaanselkä basin, chlorophyll:TP followed the same trend as in the Enonselkä basin (Fig. 6a), but the relationship was not statistically significant. Compared to the Enonselkä basin, after the intensive fish removal, the standardized chlorophyll:TP ratio in the Kajaanselkä basin indicated a consistently positive anomaly between 1995 and 2000 (Fig. 5c) which may reflect the effect of intensive fish removal in the Enonselkä basin. Because of the higher variation of the phytoplankton results, the same was not so clear in the phytoplankton biomass:TP ratio.
Relationship of chlorophyll concentration and phytoplankton biomass (with and without cyanobacteria) with total phosphorus concentration in a the Enonselkä (grey) and b Kajaanselkä (blue) basins in June –August. Panel b shows the Kajaanselkä basin data enlarged. The annual means are consecutively connected. Thick black lines between 1992 and 1993 denote the turning points due to intensive fish removal; Yellow dots – The first results; Red dots – the results from 2019; Dashed line– Linear regression of the results
The segmented linear regression analysis indicated significant breakpoints in chlorophyll concentration and phytoplankton biomass which correspond to ~ 40 mg TP m−3 (Table 2) in agreement with the stepwise decrease in nutrient concentrations between 1992 and 1993 (Fig. 6). The respective breakpoints for the Kajaanselkä basin indicated the same timing, but it was directionally significant only for chlorophyll.
To minimize the effect of regional factors on the interpretation of the nutrient and phytoplankton results, we divided the values of the Enonselkä basin by corresponding values of the Kajaanselkä basin (the opposite for Secchi depth), and these ratios were standardized. In agreement with each other, the ratios for TP, TN, chlorophyll concentration, and phytoplankton biomass indicated improvement in water quality between 1989 and 1997 (Fig. 4). After that, some retrogression happened, but the water quality to reach a stationary phase. The only exception was TN which, due to denitrification intensified by large scale oxygenation in 2010-2018, remained at low level.
Composition of phytoplankton
In the Enonselkä basin, the large interannual variation of phytoplankton biomass was mainly due to cyanobacteria (coefficient of variation 136% vs. 39–64% in the other groups). Their importance was emphasized by the fact that when excluded from the data, the statistical significance of the trend in phytoplankton biomass became weaker (r2 = 0.10, P = 0.06 with cyanobacteria; r2 = 0.04, P = 0.22 without cyanobacteria) between 1984 and 2019. After wastewater diversion, cyanobacteria formed up to 80% of phytoplankton biomass between 1979 and 1982 (Fig. 7) when the dominant taxon was Planktothrix agardhii (Gomont) Anagnostidis & Komárek (may also include the genus Phormidium), which is conspecific (Humbert & LeBerre, 2001) with Planktothrix rubescens (De Candolle ex Gomont) Anagnostidis & Komárek. In the mid-1980s, Aphanizomenon (including a small number of Cuspidothrix) mostly replaced Planktothrix (Fig. 8). Although phytoplankton biomass roughly halved, cyanobacteria still accounted for ~ 60% of the total phytoplankton biomass. At the onset of the intensive fish removal in 1989–1990, bloom-forming cyanobacteria practically disappeared for almost 10 years, and their share in phytoplankton biomass decreased to ~ 10%.
a Relative abundances of the most important phytoplankton groups in the Enonselkä and Kajaanselkä basins in June–August. Dull colours mark long sampling intervals at the beginning of the study. b Biomasses of different phytoplankton groups in the Enonselkä basin during the same period. The explanations for the colour bars are as in Fig. 1
Mean biomasses of the dominating cyanobacterial genera in the Enonselkä and Kajaanselkä basins in 1965–2019. Note the ten times higher scale of the Enonselkä basin. The vertical bar colours are as in Fig. 1; For improved comparability, the respective bars are shown in grey colours for the Kajaanselkä basin where remediation measures were not made
After 2000, bloom-forming cyanobacteria occasionally returned to develop high biomass (Figs. 7, 8). Planktothrix formed the highest proportion of phytoplankton biomass in 2002, 2006, 2016, and 2018 (Fig. 8). Microcystis spp. were mainly present between 1980 and 2000, but their biomass was never dominant. Dolichospermum (formerly Anabaena) and other cyanobacteria (particularly Woronichinia) were present in low numbers. However, in 2009 Dolichospermum formed almost as high biomass as the cyanobacteria in the late 1980s (Fig. 8).
The proportion of cyanobacteria in phytoplankton biomass was markedly lower in the Kajaanselkä basin (12%) than in the Enonselkä basin (34%) between 1984 and 2019 (Fig. 7). Despite that, the successions of Planktothrix, Aphanizomenon, and Dolichospermum were quite similar in both basins.
Diatoms were the second most important taxonomic group in the phytoplankton biomass of the Enonselkä basin, and they did not show any detectable response to intensive fish removal or oxygenation (Figs. 7 and 9). Their biomass showed no significant trend after 1992 (r = 0.07). On the contrary, in the Kajaanselkä basin, diatoms were generally dominant without a statistically significant trend during the study period. In both basins, high diatom biomass years were occasional and composed of variable taxa, without any temporal trends in species composition or biomass corresponding to cyanobacteria. There was also negligible synchrony in the occurrences of different diatom taxa between the basins. The only exception was Fragilaria crotonensis Kitton, which increased in both basins in the 1980s but decreased rather smoothly by order of magnitude towards the end of the study.
Biomass of the dominating diatom genera in the Enonselkä basin in June–August of 1965–2019. The bar colours are as in Fig. 1
Among the other phytoplankton groups in the Enonselkä basin, the decreases in dinophytes and ochrophytes were statistically significant (r = − 0.59, P = 0.001, and r = − 0.50, P = 0.008, respectively) after 1992 (Fig. 7). In addition to that, the decreasing trends of chlorophytes and cryptophytes (r = − 0.37, P = 0.06 for both) were almost significant. In the Kajaanselkä basin, the decreasing trends were significant for diatoms (r = − 0.52, P = 0.005) and ochrophytes (r = − 0.42, P = 0.03), while the decrease in cryptophytes (r = − 0.35, P = 0.07) was almost significant.
Discussion
The favorable development of the Enonselkä basin after the 1980s resulted from many concurrent factors. The dilution of past wastewaters was still approaching fulfillment (Salonen et al., 2020), and there was a decreasing trend in precipitation (Fig. 1), which meant a reduction in the nutrient load from the catchment area. The decrease in the use of fertilizers in agriculture (22% of the catchment area) and atmospheric deposition (Fig. 3) also supported the positive development of the lake. In reality, atmospheric N deposition was probably markedly higher than that shown in Fig. 3. The annual N deposition measured in 2009 at three locations on the shores of Lake Vesijärvi (Autio & Malin, 2010, unpublished report of the City of Lahti) was 1.95 times higher (CV 14%) than in the pristine forest site implying the local influence of the City of Lahti. The effects of the diversion of the wastewaters of the City of Lahti elsewhere from the Enonselkä basin were straightforwardly positive, but the effects of the additional remediation measures were complicated by the challenge of partly overlapping measures, decreasing external loading (Fig. 1), and climate change occurring in parallel. Despite seemingly small temperature changes, climate warming can enhance eutrophication directly and indirectly through various temporal and spatial processes (review of Meerhoff et al., 2022). Hecht et al. (2022) also propose that fluctuation in temperature has an impact on cyanobacterial bloom development. In the Enonselkä basin, the effects of climate warming could not be differentiated from the variation due to other factors.
Oxygenation
The implementation of oxygenation at the end of the 1970s interrupted the favorable development in chlorophyll concentration and cyanobacterial biomass. It was probably due to an increase of up to ~ 5 ℃ in temperature created by pumping warm epilimnetic water into the hypolimnion. It enhanced the ebullition of carbon dioxide and methane from the sediment, dramatically increasing nutrient release from the sediment. In the early 1980s, the ebullition caused considerable interference in hydroacoustic fish studies, but in the 2000s, no interference was found (Ruuhijärvi et al. 2020), probably due to decreased sedimentation and mineralization of legacy organic matter in the sediment. After the early oxygenation was terminated in 1984, the nutrient concentrations in deep water remained high for a few years. This was probably due to a spiral-type response (Salonen et al., 2020), where the increased primary production raised the pH of the water to values of > 11 high enough to amplify the leaching of P from sediments in shallow water.
The second, large-scale phase of oxygenation of the Enonselkä basin from 2010 to 2018 increased hypolimnetic oxygen concentration to sufficiently high levels to decrease internal loading from sediments in deeper water (Salonen et al., 2020). In winter, high oxygen concentrations could be maintained even with three pump stations, but at higher summer temperatures, up to nine pump stations in 2010–2018, operated between the establishment of summer stratification and autumnal overturn, could only delay the development of deep-water anoxia. However, the pumps could maintain the redox potential at the sediment surface high enough to prevent nitrate depletion and thus enhancement of nutrient release. Oxygenation stabilized epilimnetic TN, TP, and chlorophyll concentrations (Salonen et al., 2020).
In the literature, hypolimnetic oxygenation has generally yielded favorable results, particularly in controlling cyanobacteria (Preece et al., 2019), but in the Enonselkä basin, oxygenation in the early phase of recovery probably supported the development of cyanobacterial blooms. In two Swiss lakes (Gächter & Wehrli, 1998) and five Danish lakes (Liboriussen et al., 2009), the effect of oxygenation on epilimnetic nutrient concentration was also found negligible. Although the large-scale oxygenation in the Enonselkä basin had an apparent positive effect on deep-water oxygen and nutrient concentrations (Salonen et al., 2020), it had no pronounced impact on TP concentration and phytoplankton biomass in the epilimnion. It is reasonable because water volume below the typical ~ 10 m thickness of the epilimnion (Salonen et al., 2020) represents less than 10% of the total volume of the Enonselkä basin. Further, during the oxygenation years, TP concentration at 29 m depth did not decrease during summer stratification, while due to denitrification, TN concentration decreased by ~ 40% compared to the median of the preceding 9 years (Salonen et al., 2020).
Biomanipulation
Little attention has been paid to macrophytes and periphyton in the littoral zone of the Enonselkä basin. Under increased water transparency in the mid-1990s, macrophytes expanded their habitat from the maximum depth of 2 m to 4 m (Venetvaara & Lammi, 1995) and eventually covered almost half of the Enonselkä basin. Thus, despite the lack of more detailed information, there is no doubt that, as competitors of phytoplankton for nutrients, macrophytes also contributed to the success of the biomanipulation as strikingly demonstrated in Alte Donau (Dokulil et al., 2018), a seepage lake in Austria.
The amount of P annually removed in intensive fish removal can be estimated according to the results of Boros et al. (2012). In 1991–1993 the calculated amount corresponded to ~ 21–27% of TP in the whole water mass. This substantial decrease in P availability was inevitably significant for phytoplankton. Between 1996 and 2020, the respective percentage was lower, 6.2 ± 2.3% (mean ± SD), but also significant when TP concentration is within a range in which limitation of biomass and thus of cyanobacteria sets in.
Anttila et al. (2013) synthesized the chlorophyll and TP results of the Enonselkä basin using the approach of Bestelmeyer et al. (2011) to detect possible regime shifts. They identified a major transition (at a 10% risk level) in 1990, which does not correspond to the result of our analysis which found a clear breakpoint for 1993 in our breakpoint analysis. Instead, our results agree with their minor transitions (at a 20% risk level) of chlorophyll in 1989 and 1993. The transition in the year 1989 cannot be attributed to intensive fish removal but declining chlorophyll concentrations were due to the continuing dilution of past wastewater discharges and recovery from the preceding internal nutrient loading episode. In 1993, the abrupt reductions of TP and TN concentrations were probably responsible for the decrease in chlorophyll concentration, possibly augmented by the simultaneous decreases in atmospheric deposition of nutrients and decreased use of fertilizers in agriculture. In addition an effect of increased zooplankton grazing cannot be excluded (Anttila et al., 2013).
None of the above-discussed factors can explain the abrupt decreases in TN, TP, and chlorophyll concentrations or the increase in Secchi depth between 1992 and 1993. Horppila et al. (1998) suggested that the reduction in fish-mediated internal nutrient loading from the sediment of the Enonselkä basin was more important than the reduction in planktivory. This is because benthivorous fish fluff the sediment, which increases turbidity and enhances nutrient flux to the water (Horppila & Kairesalo, 1990, 1992). Therefore, the reduction of roach biomass (54% of the total catch) from 180 kg ha−1 to 50 kg ha−1 during the intensive fish removal (Horppila & Peltonen, 1994) was an essential factor in the nutrient dynamics of the Enonselkä basin. In addition, a shift in the age structure of the roach further sharpened the change in total nutrient and chlorophyll concentrations between 1992 and 1993. In 1994, the three youngest age groups, which eat less benthos (Horppila, 1994), accounted for about twice as much roach biomass as in 1992.
Removal of planktivorous and benthivorous fish is widely used to reduce the abundance of phytoplankton and cyanobacteria, but the results have often been contradictory (Triest et al., 2016; Paerl et al., 2016; Yin et al., 2022). Positive results may also be overrepresented because negative results are a barrier to publication. In addition, because many factors can have different short-term effects in different combinations and timing, the variation of the results in a given lake is high and can include random short-term trends. Therefore, long-term monitoring is essential for a realistic evaluation of biomanipulation results for one lake. Unfortunately, obtaining financing for comprehensive studies is challenging.
Most attention in biomanipulation studies has traditionally been paid to the predation of zooplanktivorous fish on large cladocerans, like Daphnia, which are efficient filter feeders with a high potential to control phytoplankton. Zooplankton data from the Enonselkä basin before 2000 are scarce, but there is no evidence that large Daphnia played a dominating role (Hansson et al., 1998). Other zooplankton can also mediate dynamic trophic cascades (Urrutia-Cordero et al., 2015; Ger et al., 2016). In a paleolimnological study, Nykänen et al. (2010) observed a significant ~ 2% increase in the length of Daphnia ephippia in the laminated sediment of the Enonselkä basin between 1992 and 1993, indicating increased grazing pressure at the time when TN, TP, and chlorophyll concentrations suddenly decreased. Due to the nonlinear nature of the length-to-mass relationships (Bottrell, 1976), the corresponding increase in Daphnia biomass was probably substantially higher than indicated by the linear measures of ephippia. Unfortunately, incomplete preservation and variable hydrodynamic focusing render cladoceran remains in the sediment unreliable indicators of the past population size (Nykänen et al., 2009). Nevertheless, the diets of roach and smelt (Horppila, 1994; Horppila et al., 1996) show that zooplankton was an essential component in the diet of zooplanktivorous fish. Therefore, ~ 80% removal of zooplanktivorous fish, mainly smelt (~ 30% of the total catch of the fish removal) and roach (Horppila et al., 1998) no doubt affected zooplankton biomass and composition. The migration of roaches to the littoral area to avoid predation (Peltonen et al., 1996, 1999) further emphasized the role of smelt. Notably, the biomass of strictly zooplanktivorous smelt also halved between 1992 and 1993 (Horppila et al., 1996) when TN, TP, and chlorophyll concentrations suddenly decreased.
Chlorophyll:TP ratio can reflect the intensity of zooplankton grazing on phytoplankton (e.g., Mazumder, 1994; Yin et al., 2022), but it is also affected by many other factors, and lake-specific differences can be substantial (Yuan & Jones, 2020). As expected, chlorophyll:TP and phytoplankton biomass:TP ratios in the Enonselkä basin decreased during and soon after the intensive fish removal (Fig. 5), indicating increasing grazing on phytoplankton. However, in 2015–2017, extremely high (up to > 100 kg ha−1) smelt biomass (Ruuhijärvi et al., 2020) did not result in high (0.35–0.41) chlorophyll:TP ratio and phytoplankton biomass:TP ratios. Further, in 2011, when high water temperatures destroyed most of the smelt population (Ruuhijärvi et al., 2020), both ratios were not different from those in 2015–2017. This discrepancy is difficult to explain, but possibly effects were missed due to too infrequent (monthly) phytoplankton sampling frequency. The similar developments of chlorophyll:TP ratios in the Enonselkä and Kajaanselkä basins, where fish removal was almost negligible, suggest that it may not be an unambiguous indicator of a trophic cascade. Although fish are motile, the fish population in the much larger Kajaanselkä basin was not markedly affected.
The results from the Enonselkä basin were quite opposite to those known from Lake Ringsjön, in Sweden (Ekvall et al. (2014), which is only slightly larger (surface area 40 km2) than the Enonselkä basin. Fish removal in Lake Ringsjön, initially ~ 25 kg ha−1 year−1, decreased in 3 years to a relatively steady level of ~ 5 kg ha−1 year−1. In June, the proportion of Daphnia in zooplankton biomass roughly triplicated in 7 years and at the same time, cyanobacterial biomass declined to a quarter. Although the fish removal rates were only a quarter of those in the Enonselkä basin, the effect of Daphnia on cyanobacteria in Lake Ringsjön was apparent. However, these authors reported no similar effects in July and August. The contrasting results between summer months suggest that the outcome of fish removal may be seasonally different and achieved through different pathways in the food web.
In 70 Danish and Dutch lakes (area 0.002 km2–9.4 km2), from which more than 50% of zooplanktivorous fish were removed within 1–3 years (Søndergaard et al., 2007), the positive effect of biomanipulation did not last longer than ~ 10 years. On the contrary, in the Enonselkä basin, the positive impact has lasted for > 30 years. The most likely explanation for the difference is the continuous medium-intensity fishing at the Enonselkä basin after the intensive fish removal.
Dynamics of phytoplankton during the recovery process
During the last half of the 1980s, Aphanizomenon replaced Planktothrix in the Enonselkä basin. Although the biomass of Aphanizomenon was lower, as a surface scum-forming taxon its harmful effect on the recreational value of the lake remained high. The rapid shift from the dominance of non-nitrogen-fixing Planktothrix to nitrogen-fixing Aphanizomenon was probably due to different responses to fluctuating hydrodynamics and, perhaps most likely, to the decreases in nutrient concentrations because TN:TP ratio in the epilimnion was high and rather stable (Fig. 5). Planktothrix can be present throughout the water column or in the metalimnion (Van Liere & Mur, 1980; Jacquet et al., 2005; Posch et al., 2012), and the taxon tolerates lower availability of light than many other cyanobacteria (Dokulil & Teubner, 2000, 2012). In the Enonselkä basin, this was manifested by the red colour of the phycoerythrin-rich Planktothrix during winters until 1985 (Keto & Tallberg, 2000). Between 1978 and 1985, the oxygen concentration in the water column below 12–16 m depth was hypoxic in late summer, while during the following 10 years, the hypoxic zone was below ~ 20 m depth (Salmi et al., 2014; Salonen et al., 2020). The increase in stratification depth possibly reduced light availability in the nutricline to such a low level that Planktothrix could no longer effectively utilize higher metalimnetic P concentration. Shading by surface blooming cyanobacteria (e.g., Mur, 1999) probably also favored Aphanizomenon over Planktothrix. Because the epilimnetic TN concentration was high and relatively stable, nitrogen fixation hardly provided an advantage to Aphanizomenon. The current TP concentration in the Enonselkä basin has not yet reached the critical levels for dominating cyanobacteria (Planktothrix ~ 15 mg m−3, Aphanizomenon 15–24 mg m−3, Microcystis > 20 mg m−3, and Dolichospermum 24 mg m−3) found by Vuorio et al. (2020) in > 2000 Finnish lakes.
In the 2000s, the increasing trend in chlorophyll concentration and phytoplankton biomass (Fig. 4) as well as their variation (Fig. 5) in the Enonselkä basin were likely due to the decrease in fish removal (Fig. 3) and changes in weather-related factors. Simultaneously increasing summer precipitation (i.e. increasing external nutrient loading) (Fig. 1) and weak oxygen conditions in the hypolimnion contributed to nutrient availability in the epilimnion, but their relative importance is unknown. Distinct cyanobacterial blooms were observed only in 5 years out of the 25 last study years (Fig. 8), while in the Kajaanselkä basin they occurred only once. Blooms can appear and occasionally disappear during recovery without being triggered by high nutrient concentrations. One of the most striking examples is Lake Bourget in France, where Planktothrix rubescens blooms appeared after more than 20 years of oligotrophication (Jacquet et al., 2005) when the epilimnetic TP concentration was close (26 mg m−3) to that in the Enonselkä basin today. In Lake Bourget the blooming period lasted 15 years, but the species practically disappeared for 6 years until it reappeared (Moiron et al., 2021).
Cyanobacteria thrive at a wide range of nutrient concentrations, including oligotrophic waters (Reinl et al., 2021), but high abundances are most typically associated with high TP concentrations (Verspagen et al, 2022) unless N is limiting (Shatwell & Köhler, 2019). Heavy cyanobacterial blooms may occur relatively frequently in the early phase of the recovery from eutrophication, and decreasing nutrient availability gradually decreases their occurrence (Salmaso & Tolotti, 2021). The critical TP concentration at which the contribution of cyanobacteria becomes dominant may be as high as ~ 50–90 mg m−3 (Chorus et al., 2021), which corresponds to the range of the concentrations prevailing in the Enonselkä basin between 1975 and 1980. The ~ 13% reduction of the TP concentration from ~ 31 mg m−3 to 27 mg m−3 between 1992 and 1993 was probably a critical change related to the risk of cyanobacterial blooms. At TP concentrations < 10–20 mg m−3 (Chorus et al., 2000), massive cyanobacterial blooms are unlikely, which agrees with the Finnish lake data (Vuorio et al., 2020). Fastner et al. (2016) suggested a higher critical limit of < 20–50 mg m−3 for eight lakes ranging from 0.42 km2 to 536 km2. They also suggested that critical concentration for controlling cyanobacteria may depend on the thickness of the epilimnion, with a higher threshold when it is thin. In deep and stratifying Mondsee, Dokulil & Jagsch (1992) found that the decrease of TP from 25–30 mg m–3 to 10 mg m−3 led to the disappearance of Planktothrix rubescens. The low occurrence of harmful cyanobacteria in the Kajaanselkä basin (TP concentration ~ 15 mg m−3 at the end of this study) is in line with the above results. However, the mean TP concentration of 27 mg m−3 in the Enonselkä basin in June–August after 1993 is still sufficiently high for cyanobacterial blooms, calling for a further reduction to reliably prevent them.
Jeppesen et al. (2005) found that after reduced nutrient loading, chrysophytes and dinophytes in deep lakes became more dominant at the expense of cyanobacteria, whereas in shallow lakes, the relative importance of diatoms, cryptophytes, and chrysophytes increased. However, the target of restoration is not only to decrease the relative proportion of cyanobacteria, but also total phytoplankton biomass. In the Enonselkä basin, the biomass of almost all phytoplankton groups decreased after the intensive fish removal, reflecting decreasing trophic state. Because quite similar development occurred in the Kajaanselkä basin, although this was not oxygenated and fish removal at best influenced this indirectly via exchange with the Enonselkä basin, the changes after the 1980s cannot be unambiguously attributed to intensive fish removal and oxygenation.
Implications for future
The remediation measures (excluding the ongoing ~ 50% diversion of urban runoff) applied in the Enonselkä basin may have reached practical limits to a further decrease in nutrient concentrations and phytoplankton biomass. Although the frequency of cyanobacterial blooms has substantially declined, when they do occur, their levels of biomass are still of concern, and as the potentials of the remediation measures applied so far are limited, new alternatives are needed. Reduction of external loading continues to be the primary goal, but it is economically challenging, and rapid results are not likely. Chemical immobilization of P in the sediment is one possibility, but it is too expensive for lakes of the size of the Enonselkä basin and includes potential risks to the lake biota. Chemical remediation measures usually also target the adsorption of nutrients in the sediment and long-term, if not the "final" solution to the problem. Methods targeting the control of existing cyanobacterial blooms have had less attention and they do not have an established place in the repertoire of water managers. For example, sonification and hydrogen peroxide treatments (Lürling et al., 2014) have been suggested, but they are also incompatible with the scale of large water bodies. Lürling & van Oosterhout (2013) used a combination of flocculent (poly-aluminium chloride, PAC) and solid phase P-sorbent (Phoslock) to effectively sediment developing cyanobacterial bloom. Based on the positive results, Drummond et al. (2022) encouraged temporal and spatial trials of this technique to find the best practices for in-lake measures to reduce prevailing cyanobacterial blooms. McKercher et al. (2022) also successfully used continuous application of lanthanum chloride to maintain low soluble reactive phosphorus concentration in a eutrophic pond.
Because the frequency of cyanobacterial blooms in the Enonselkä basin is modest and TN:TP ratio in the epilimnion was typically 30–40, on-demand interception of the growth of cyanobacteria before they develop harmful blooms is potentially cost-effective. For lakes that are no longer in the phase of continuous cyanobacterial blooming, we suggest testing a simple approach combining the ideas of Lürling & van Oosterhout (2013) and McKercher et al. (2022). In the Enonselkä basin, existing and continuously operated phycocyanin sensors may be used to detect a threshold to start a low-dose PAC treatment to precipitate inorganic P in the epilimnion. Considering only ~ 20% annual probability of pronounced cyanobacterial blooms in the Enonselkä basin, the number of treatments would remain small. Because orders of magnitude less chemical would be needed compared to classical treatments, both economic and environmental challenges could be resolved. If the micro-floc technique (Moore et al., 2009) is applied to spread chemicals from a boat, the precipitate settling should be slow, and hydrodynamics could adequately mix the chemical into the larger body of water. Low PAC doses may also be relatively more efficient in binding P than traditionally used high doses (de Vicente et al., 2008; Jensen et al., 2015). If sedimentation of cyanobacterial cells is incomplete, perhaps the greatest challenge to the interception is the ability of cyanobacteria to store P (Carey et al., 2012).
The results of the Enonselkä and Kajaanselkä basins suggest that TN and TP concentrations provide robust approaches for monitoring the development of the trophic status of the lake also in the future. The simple Secchi depth is also useful, but our results suggest caution. The ~ 50% increase in the Secchi depth of the Kajaanselkä basin between 1992 and 1997 compared with the ~ 70% increase in the Enonselkä basin seems too high to be explained only by reduced nutrient inflow from the Enonselkä basin. Thus, regional conditions that both basins have in common probably also contributed to the favorable development of the Enonselkä basin during biomanipulation. This possibility is corroborated by weather data (Fig. 1).
Phytoplankton analysis is a valuable but tedious and expensive monitoring tool. However, the present technological advances have not yet been transferred into current practices. As Wagner & Adrian (2011) demonstrated, monthly sampling is too infrequent to capture the dynamics of phytoplankton assemblage, and even significant blooms may remain undetected. The results from Lake Vesijärvi (Horppila et al., 1998; Anttila et al., 2008) have also verified that horizontally and vertically differentiated distributions may result in high variation of the phytoplankton results. The problem could be solved with the present technology. Composite samples could be automatically collected (for example, monthly) so that temporal fluctuations of phytoplankton could be averaged. Then, phytoplankton species composition would be truly representative, and confidence limits of the summer months would provide unprecedented reliability to interpret results. A tremendous improvement could be reached with a modest investment.
Conclusions
The diversion of wastewaters elsewhere was a turning point in the oligotrophication of the Enonselkä basin, but the later remediation measures also had significant impacts. Oxygenation alleviated hypolimnetic hypoxia and anoxia, but its effect on nutrients was too small to reduce phytoplankton biomass significantly. On the contrary, in the most eutrophic state of the lake, it even amplified internal loading, probably chiefly through the physical disturbance it caused through the ebullition of carbon dioxide and methane. The impacts of wastewater diversion and biomanipulation on TN, TP, and chlorophyll concentration were impressive. An order of magnitude reduction in the P load to the Enonsekä basin in 1976 led to ~ 60% and biomanipulation to further ~ 30% decrease in TP concentrations. The rapid and persistent declines in TN, TP, and chlorophyll concentrations in 1993 leave no doubt that those were due to biomanipulation. The main advantage of the decrease in nutrient concentrations was reducing the cyanobacterial blooms to a fraction of the earlier frequency. One of the most interesting, albeit laborious, future challenges is to investigate what intensity of fish removal, if any, is still needed to maintain the present situation of the Enonselkä basin.
Data availability
The weather data is available from the service of the Finnish Environment Institute (https://www.ilmatieteenlaitos.fi/havaintojen-lataus). The results of physical and chemical determinations (https://wwwp2.ymparisto.fi/vesla/Common/rules/SearchRules.aspx), as well as phytoplankton (https://wwwp2.ymparisto.fi/kplank/Common/rules/SearchRules.aspx), can be loaded from the open data service of the Finnish Environment Institute. Statistics of fertilizers used in agriculture in Finland are compiled by the Natural Resources Institute Finland (statdb.luke.fi/PXWeb/pxweb/en/LUKE/LUKE__08%20Indikaattorit__06%20Ympäristö__12%20Typpi-%20ja%20fosforitase/02_Kasviravinteiden_myynti.px/?rxid=e119108e-bd95-43bf-b02c-a5f4ff9eef72).
References
Anttila, S., T. Kairesalo & P. Pellikka, 2008. A feasible method to assess inaccuracy caused by patchiness in water quality monitoring. Environmental Monitoring and Assessment 142: 11–22. https://doi.org/10.1007/s10661-007-9904-y.
Anttila, S., M. Ketola, K. Kuoppamäki & T. Kairesalo, 2013. Identification of a biomanipulation-driven regime shift in Lake Vesijärvi: implications for lake management. Freshwater Biology 58: 1494–1502. https://doi.org/10.1111/fwb.12150.
Bestelmeyer, B. T., A. Ellison, W. Fraser, K. Gorman, S. Holbrook, C. Laney, et al., 2011. Analysis of abrupt transitions in ecological systems. Ecosphere 2: 129. https://doi.org/10.1890/ES11-00216.1.
Bormans, M., B. Maršálek & D. Jančula, 2016. Controlling internal phosphorus loading in lakes by physical methods to reduce cyanobacterial blooms — a review. Aquatic Ecology 50: 407–422. https://doi.org/10.1007/s10452-015-9564-x.
Boros, G., J. Jyväsjärvi, P. Takács, A. Mozsár, I. Tátrai, M. Søndergaard & R. I. Jones, 2012. Between-lake variation in the elemental composition of roach (Rutilus rutilus L.). Aquatic Ecology 46: 385–394. https://doi.org/10.1007/s10452-012-9402-3.
Bottrell, H. H., A. Duncan, Z. M. Gliwicz, E. Grygierek, A. Herzig, A. Hillbricht-Ilkowska, H. Kurosawa, P. Larsson & T. Weglenska, 1976. A review of some problems in zooplankton production studies. Norwegian Journal of Zoology 24: 419–456.
Brett, M. T. & C. R. Goldman, 1996. A meta-analysis of the freshwater trophic cascade. Proceedings of the National Academy of Sciences 93: 7723–7726. https://doi.org/10.1073/pnas.93.15.7723.
Carey, C. C., B. W. Ibelings, E. P. Hoffmann, D. P. Hamilton & J. D. Brookes, 2012. Ecophysiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Research. 46: 1394–1407. https://doi.org/10.1016/j.watres.2011.12.016.
Carpenter, S. R., J. F. Kitchell & J. R. Hodgson, 1985. Cascading trophic interactions and lake productivity. Bioscience 35: 634–639. https://doi.org/10.2307/1309989.
Carvalho, L., S. Poikane, A. Lyche-Solheim, G. Phillips, G. Borics, J. Catalan, C. De Hoyos, S. Drakare, B. Dudley, M. Järvinen, C. Laplace-Treyture, K. Maileht, C. McDonald, U. Mischke, J. Moe, G. Morabito, P. Nõges, T. Nõges, I. A. OttPasztalenie, C. B. Skjelbred & S. Thackeray, 2012. Strength and uncertainty of phytoplankton metrics for assessing eutrophication impacts in lakes. Hydrobiologia 704: 127–140. https://doi.org/10.1007/s10750-012-1344-1.
Chakraborty, A., G. K. Saha & G. Aditya, 2022. Macroinvertebrates as engineers for bioturbation in freshwater ecosystem. Environmental Science and Pollution Research 29: 64447–64468. https://doi.org/10.1007/s11356-022-22030-y.
Chorus, I., I. R. Falconer, H. J. Salas & J. Bartram, 2000. Health risks caused by freshwater cyanobacteria in recreational waters. Journal of Toxicology and Environmental Health 3: 323–347. https://doi.org/10.1080/109374000436364.
Chorus, I., A. Köhler, C. Beulker, J. Fastner, K. van de Weyer, T. Hegewald & M. Hupfer, 2020. Decades needed for ecosystem components to respond to a sharp and drastic phosphorus load reduction. Hydrobiologia 847: 4621–4651. https://doi.org/10.1007/s10750-020-04450-4.
Chorus, I., J. Fastner & M. Welker, 2021. Cyanobacteria and cyanotoxins in a changing environment: concepts, controversies, challenges. Water 13: 2463. https://doi.org/10.3390/w13182463.
de Vicente, I., P. Huang, F. Ø. Andersen & H. S. Jensen, 2008. Phosphate adsorption by fresh and aged aluminum hydroxide. Consequences for lake restoration. Environmental Science and Technology 42: 6650–6655. https://doi.org/10.1021/es800503s.
Dokulil, M. T. & A. Jagsch, 1992. The effects of reduced phosphorus and nitrogen loading on phytoplankton in Mondsee. Austria. Hydrobiologia 243(244): 389–394. https://doi.org/10.1007/BF00007055.
Dokulil, M. T. & K. Teubner, 2000. Cyanobacterial dominance in lakes. Hydrobiologia 438: 1–12. https://doi.org/10.1023/A:1004155810302.
Dokulil, M. & K. Teubner, 2012. Deep living Planktothrix rubescens modulated by environmental constraints and climate forcing. Hydrobiologia 698: 29–46. https://doi.org/10.1007/978-94-007-5790-5_4.
Dokulil, M. T., K. Donabaum & K. Teubner (eds), 2018. The Alte Donau: Successful restoration and sustainable management – An ecosystem case study of a shallow urban lake. Springer, https://doi.org/10.1007/978-3-319-93270-5_1.
Drummond, E., V. B. G. Leite, N. P. Noyma, L. de Magalhães, C. Graco-Roza, V. L. Huszar, M. Lürling & M. M. Marinho, 2022. Temporal and spatial variation in the efficiency of a Floc & Sink technique for controlling cyanobacterial blooms in a tropical reservoir. Harmful Algae 117: 102262. https://doi.org/10.1016/j.hal.2022.102262.
Ekvall, M. K., P. Urrutia-Cordero & L.-A. Hansson, 2014. Linking cascading effects of fish predation and zooplankton grazing to reduced cyanobacterial biomass and toxin levels following biomanipulation. PLoS One 9: e112956. https://doi.org/10.1371/journal.pone.0112956.
Fastner, J., S. Abella, A. Litt, G. Morabito, L. Vörös, K. Pálffy, D. Straile, R. Kümmerlin, D. Matthews, M. G. Phillips & I. Chorus, 2016. Combating cyanobacterial proliferation by avoiding or treating inflows with high P load – experiences from eight case studies. Aquatic Ecology 50: 367–383. https://doi.org/10.1007/s10452-015-9558-8.
Gächter, R. & B. Wehrli, 1998. Ten years of artificial mixing and oxygenation: no effect on the internal phosphorus loading of two eutrophic lakes. Environmental Science and Technology 32: 3659–3665. https://doi.org/10.1021/es980418l.
Ger, K. A., P. Urrutia-Cordero, P. C. Frost, L. A. Hansson, O. Sarnelle, A. E. Wilson & M. Lürling, 2016. The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 54: 128–144. https://doi.org/10.1016/j.hal.2015.12.005.
Hansson, L.-A., H. Annadotter, E. Bergman, S. F. Hamrin, E. Jeppesen, T. Kairesalo, E. Luokkanen, P. -Å. Nilsson, M. Søndergaard & J. Strand, 1998. Biomanipulation as an application of food chain theory: constraints, synthesis and recommendations for temperate lakes. Ecosystems 1: 558–574. https://doi.org/10.1007/s100219900051.
Hecht, J. S., A. Zia, P. J. Clemins, A. W. Schroth, J. M. Winter, P. D. Oikonomou & D. M. Rizzo, 2022. Modeling the sensitivity of cyanobacteria blooms to plausible changes in precipitation and air temperature variability. Science of The Total Environment. https://doi.org/10.1016/j.scitotenv.2021.151586.
Horppila, J., 1994. The diet of roach (Rutilus rutilus (L.)) in Lake Vesijärvi and possible changes in the course of biomanipulation. Hydrobiologia 294: 35–41. https://doi.org/10.1007/BF00017623.
Horppila, J. & T. Kairesalo, 1990. A fading recovery: the role of roach (Rutilus rutilus L.) in maintaining high phytoplankton productivity and biomass in Lake Vesijarvi, southern Finland. Hydrobiologia 200(201): 153–165. https://doi.org/10.1007/978-94-017-0924-8_13.
Horppila, J. & T. Kairesalo, 1992. Impacts of bleak (Alburnus alburnus) and roach (Rutilus rutilus) on water quality, sedimentation and internal nutrient loading. Hydrobiologia 243: 323–331. https://doi.org/10.1007/978-94-011-2745-5_33.
Horppila, J. & H. Peltonen, 1994. The fate of a roach (Rutilus rutilus) stock under an extremely strong fishing pressure and its predicted development after the cessation of mass removal. Journal of Fish Biology 45: 777–786. https://doi.org/10.1111/j.1095-8649.1994.tb00943.x.
Horppila, J., K. Nyberg, H. Peltonen & T. Turunen, 1996. Effects of five years of intensive trawling on a previously unexploited smelt stock. Journal of Fish Biology 49: 329–340. https://doi.org/10.1111/j.1095-8649.1996.tb00027.x.
Horppila, J., H. Peltonen, T. Malinen, E. Luokkanen & T. Kairesalo, 1998. Top-down or bottom-up effects by fish: issues of concern in biomanipulation of lakes. Restoration Ecology 6: 20–28. https://doi.org/10.1046/j.1526-100x.1998.00613.x.
Humbert, J.-F. & B. LeBerre, 2001. Genetic diversity in two species of freshwater cyanobacteria, Planktothrix (Oscillatoria) rubescens and P. agardhii. Archiv Für Hydrobiologie 150: 197–206. https://doi.org/10.1127/archiv-hydrobiol/150/2001/197.
Hupfer, M. & J. Lewandowski, 2008. Oxygen controls phosphorus release from lake sediments – a long lasting paradigm in limnology. International Review of Hydrobiology 93: 415–432. https://doi.org/10.1002/iroh.200711054.
Jacquet, S., J. F. Briand, C. Leboulanger, C. Avois-Jacquet, L. Oberhaus, B. Tassin, B. Vinçon-Leite, G. Paolini, J.-C. Druart, O. Anneville & J.-F., Humbert, 2005. The proliferation of the toxic cyanobacterium Planktothrix rubescens following restoration of the largest natural French lake (Lac du Bourget). Harmful Algae 4: 651–672. https://doi.org/10.1016/j.hal.2003.12.006.
Jensen, H. S., K. Reitzel & S. Egemose, 2015. Evaluation of aluminum treatment efficiency on water quality and internal phosphorus cycling in six Danish lakes. Hydrobiologia 751: 189–199. https://doi.org/10.1007/s10750-015-2186-4.
Jeppesen, E., M. Søndergaard, J. P. Jensen, K. Havens, O. Anneville, L. Carvalho, M. F. Coveney, R. Deneke, M. T. Dokulil, B. Foy, D. Gerdeaux, S. E. Hampton, S. Hilt, K. Kangur, J. Köhler, E. Lammens, T. L. Lauridsen, M. Manca, R. Miracle, B. Moss, P. Nöges, G. Persson, G. Phillips, R. Portielje, S. Romo, C. L. Schelske, D. Straile, I. Tatrai, E. Willén & M. Winder, 2005. Lakes’ response to reduced nutrient loading– an analysis of contemporary data from 35 European and North American long term studies. Freshwater Biology 50: 1747–1771. https://doi.org/10.1111/j.1365-2427.2005.01415.x.
Kairesalo, T., S. Laine, E. Luokkanen, T. Malinen & J. Keto, 1999. Direct and indirect mechanisms behind successful biomanipulation. Hydrobiologia 395(396): 99–106. https://doi.org/10.1007/978-94-017-3282-6_9.
Keto, J. & I. Sammalkorpi, 1988. A fading recovery: a conceptual model for Lake Vesijärvi management and research. Aqua Fennica 18: 193–204.
Keto, J. & P. Tallberg, 2000. The recovery of Vesijärvi, a lake in southern Finland: water quality and phytoplankton interpretations. Boreal Environment Research 5: 15–26. https://doi.org/10.1080/03680770.2005.11902051.
Keto, J., P. Tallberg, I. Malin, P. Vääränen & K. Vakkilainen, 2005. The horizon of hope for L. Vesijärvi. Verhandlungen Der Internationale Vereinigung Für Theoretische Und Angewandte Limnologie 30: 448–452. https://doi.org/10.1080/03680770.2005.11902051.
Lappalainen, K. M., 1994. Positive changes in oxygen and nutrient contents in two Finnish lakes induced by Mixox hypolimnetic oxygenation method. Verhandlungen Der Internationale Vereinigung Für Theoretische Und Angewandte Limnologie 25: 2510–2513. https://doi.org/10.1080/03680770.1992.11900682.
Liboriussen, L., M. Søndergaard, E. Jeppesen, I. Thorsgaard, S. Grünfeld, T. S. Jakobsen & K. Hansen, 2009. Effects of hypolimnetic oxygenation on water quality: results from five Danish lakes. Hydrobiologia 625: 157–172. https://doi.org/10.1007/s10750-009-9705-0.
Lürling, M. & F. van Oosterhout, 2013. Controlling eutrophication by combined bloom precipitation and sediment phosphorus inactivation. Water Research 47: 6527–6537. https://doi.org/10.1016/j.watres.2013.08.019.
Lürling, M., D. Meng & E. Faassen, 2014. Effects of hydrogen peroxide and ultrasound on biomass reduction and toxin release in the cyanobacterium, Microcystis aeruginosa. Toxins 6: 3260–3280. https://doi.org/10.3390/toxins6123260.
Mazumder, A., 1994. Phosphorus-chlorophyll relationships under contrasting herbivory and thermal stratification: predictions and patterns. Canadian Journal of Fisheries and Aquatic Sciences 51: 390–400. https://doi.org/10.1139/f94-040.
McKercher, L. J., T. L. Messer, A. R. Mittelstet & S. D. Comfort, 2022. A biological and chemical approach to restoring water quality: a case study in an urban eutrophic pond. Journal of Environmental Management 318: 11543. https://doi.org/10.1016/j.jenvman.2022.115463.
Meerhoff, M., J. T. A. AudetDavidson, L. De Meester, S. Hilt, S. Kosten, Z. Liu, N. Mazzeo, H. Paerl, M. Scheffer & E. Jeppesen, 2022. Feedbacks between climate change and eutrophication: revisiting the allied attack concept and how to strike back. Inland Waters 12: 1–42. https://doi.org/10.1080/20442041.20222029317.
Moiron, M., F. Rimet, C. Girel & S. Jacquet, 2021. Die hard in Lake Bourget! The case of Planktothrix rubescens reborn. International Journal of Limnology 57: 19. https://doi.org/10.1051/limn/2021014.
Moore, B. C., D. Christensen & A. C. Richter, 2009. Newman Lake restoration: A case study. Part II. Microfloc alum injection. Lake and Reservoir Management 25: 351–363. https://doi.org/10.1080/07438140903172923.
Mur, L. R., O. M. Skulberg & H. Utkilen, 1999. Cyanobacteria in the environment. In: Chorus, I. & J. Bartram (Eds), Toxic Cyanobacteria in Water: A Guide to Their Public Health, Consequences, Monitoring and Management. St. Edmundsbury Press, Suffolk, pp. 15–40, https://doi.org/10.1201/9781482295061-8
Nykänen, M., K. Vakkilainen, M. Liukkonen & T. Kairesalo, 2009. Cladoceran remains in lake sediments: a comparison between plankton counts and sediment records. Journal of Paleolimnology 4: 551–570. https://doi.org/10.1007/s10933-008-9304-5.
Nykänen, M., T. Malinen, K. Vakkilainen, M. Liukkonen & T. Kairesalo, 2010. Cladoceran community responses to biomanipulation and re-oligotrophication in Lake Vesijärvi, Finland, as inferred from remains in annually laminated sediment. Freshwater Biology 55: 1164–1181. https://doi.org/10.1111/j.1365-2427.2009.02341.x.
Orihel, D. M., H. M. Baulch, N. J. Casson, R. L. North, C. T. Parsons, D. C. M. Seckar & J. J. Venkiteswaran, 2017. Internal phosphorus loading in Canadian fresh waters: a critical review and data analysis. Canadian Journal of Fisheries and Aquatic Sciences 74: 2005–2029. https://doi.org/10.1139/cjfas-2016-0500.
Paerl, H. W., W. S. Gardner, K. E. Havens, A. R. Joyner, M. J. McCarthy, S. E. Newell, B. Qin & J. T. Scott, 2016. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 54: 213–222. https://doi.org/10.1016/j.hal.2015.09.009.
Peltonen, H., H. Rita & J. Ruuhijärvi, 1996. Diet and prey selection of pikeperch (Stizostedion lucioperca (L.)) in Lake Vesijärvi analysed with a logit model. Annales Zoologici Fennici 33: 481–487.
Peltonen, H., J. Ruuhijärvi, T. Malinen, J. Horppila, M. Olin & J. Keto, 1999. The effects of food-web management on fish assemblage dynamics in a north temperate lake. Journal of Fish Biology 55: 54–67. https://doi.org/10.1111/j.1095-8649.1999.tb00656.x.
Petr, T., 2000. Interactions between fish and aquatic macrophytes in inland waters. A review. 185 pp. In: FAO Fisheries Technical Paper, No. 396, FAO, Rome, Italy.
Posch, T., O. Köster, M. M. Salcher & J. Pernthaler, 2012. Harmful filamentous cyanobacteria favoured by reduced water turnover with lake warming. Nature Climate Change 2: 809–813. https://doi.org/10.1038/nclimate1581.
Prairie, Y. T., C. de Montigny & P. A. Del Giorgio, 2001. Anaerobic phosphorus release from sediments: a paradigm revisited. Verhandlungen Der Internationale Vereinigung Für Theoretische Und Angewandte Limnologie 27: 4013–4020.
Preece, E. P., B. C. Moore, M. M. Skinner, A. Child & S. Dent, 2019. A review of the biological and chemical effects of hypolimnetic oxygenation. Lake and Reservoir Management 35: 229–246. https://doi.org/10.1080/10402381.2019.1580325.
Reinl, K. L., J. D. Brookes, C. C. Carey, T. D. Harris, B. W. Ibelings, A. M. Morales-Williams, L. N. De Senerpont Domis, K. S. Atkins, P. D. F. Isles, J. P. Mesman, R. L. North, L. G. Rudstam, J. A. A. Stelzer, J. J. Venkiteswaran, K. Yokota & Q. Zhan, 2021. Cyanobacterial blooms in oligotrophic lakes: Shifting the high-nutrient paradigm. Freshwater Biology 66: 1846–1859. https://doi.org/10.1111/fwb.13791.
Ruuhijärvi, J., T. Malinen, K. Kuoppamäki, P. Ala-Opas & M. Vinni, 2020. Responses of food web to hypolimnetic aeration in Lake Vesijärvi. Hydrobiologia 847: 4503–4523. https://doi.org/10.1007/s10750-020-04319-6.
Salmaso, N. & M. Tolotti, 2021. Phytoplankton and anthropogenic changes in pelagic environments. Hydrobiologia 848: 251–284. https://doi.org/10.1007/s10750-020-04323-w.
Salmi, P., I. Malin & K. Salonen, 2014. Pumping of epilimnetic water into hypolimnion improves oxygen but not necessarily nutrient conditions in a lake recovering from eutrophication. Inland Waters 4: 425–434. https://doi.org/10.5268/IW-4.4.631.
Salonen, K., J. Sarvala, J. Horppila, J. Keto, I. Malin, T. Malinen, J. Niemistö & J. Ruuhijärvi, 2020. Development of Lake Vesijärvi through four decades of remediation efforts. Hydrobiologia 847: 4601–4619. https://doi.org/10.1007/s10750-020-04338-3.
Scheffer, M., S. H. Hosper, M.-L. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology & Evolution 8: 275–279. https://doi.org/10.1016/0169-5347(93)90254-M.
Shatwell, T. & J. Köhler, 2019. Decreased nitrogen loading controls summer cyanobacterial blooms without promoting nitrogen-fixing taxa: Long-term response of a shallow lake. Limnology and Oceanography 64: S166–S178. https://doi.org/10.1002/lno.11002.
Søndergaard, M., E. Jeppesen, T. L. Lauridsen, C. Skov, E. H. Van Nes, R. Roijackers, E. Lammens & R. Portielje, 2007. Lake restoration: successes, failures and long-term effects. Journal of Applied Ecology 44: 1095–1105. https://doi.org/10.1111/j.1365-2664.2007.01363.x.
Svirčev, Z., D. Lalić, G. B. Savić, N. Tokodi, D. D. Backović, L. Chen, J. Meriluoto & G. A. Codd, 2019. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Archives of Toxicology 93: 2429–2481. https://doi.org/10.1007/s00204-019-02524-4.
Triest, L., I. Stiers & S. Van Onsem, 2016. Biomanipulation as a nature-based solution to reduce cyanobacterial blooms. Aquatic Ecology 50: 461–483. https://doi.org/10.1007/s10452-015-9548-x.
Urrutia-Cordero, P., M. K. Ekvall & L.-A. Hansson, 2015. Response of cyanobacteria to herbivorous zooplankton across predator regimes: who mows the bloom? Freshwater Biology 60: 960–972. https://doi.org/10.1111/fwb.12555.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitteilungen - Internationale Vereinigung Der Theoretische Und Angewandte Limnologie 9: 1–38.
Van Liere, L. & L. R. Mur, 1980. Occurrence of Oscillatoria agardhii and some related species, a survey. Developments in Hydrobiology 2: 57–77.
Venetvaara, J. & E. Lammi, 1995. The current state and recent changes in the macrophytic vegetation of Lake Vesijärvi (in Finnish with English abstract). In Sammalkorpi, I., J. Keto, T. Kairesalo, E. Luokkanen, M. Mäkelä, J. Vääriskoski & E. Lammi (Eds), Lake Vesijärvi Project: Mass Removal of Cyprinids and Traditional Water Protection. Publications of Water and Environment Administration, Finnish Environment Institute – Series A 218: 101–106.
Verspagen, J. M. H., Xi. Ji, Q.-X. Liu & J. Huisman, 2022. Large-scale variation in phytoplankton community composition of >1,000 lakes across the U.S.A. Environmental Research: Ecology: 015001 https://doi.org/10.1088/2752-664X/ac788c.
Vuorio, K., M. Järvinen & N. Kotamäki, 2020. Phosphorus thresholds for bloom-forming cyanobacterial taxa in boreal lakes. Hydrobiologia 847: 4389–4400. https://doi.org/10.1007/s10750-019-04161-5.
Wagner, C. & R. Adrian, 2011. Consequences of changes in thermal regime for plankton diversity and trait composition in a polymictic lake: a matter of temporal scale. Freshwater Biology 56: 1949–1961. https://doi.org/10.1111/j.1365-2427.2011.02623.x.
Yin, C., W. He, L. Guo, L. Gong, Y. Yang, J. Yang, L. Ni, Y. Chen & E. Jeppesen, 2022. Can top-down effects of planktivorous fish removal be used to mitigate cyanobacterial blooms in large subtropical highland lakes? Water Research 218, https://doi.org/10.1016/j.watres.2022.118483.
Yuan, L. L. & J. R. Jones, 2020. Rethinking phosphorus chlorophyll relationships in lakes. Limnology Oceanography 65: 1847–1857. https://doi.org/10.1002/lno.11422.
Acknowledgements
The City of Lahti initiated and implemented the monitoring program and provided an attractive environment for various research projects. This report is an outcome of the Lahti Lakes 2021 Symposium. Lake Vesijärvi Foundation greatly supported the Lake Vesijärvi research. We thank anonymous reviewers for their thorough and constructive comments.
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital. Open access funding was provided by the University of Helsinki. The monitoring of Lake Vesijärvi was financed by the City of Lahti Environmental Services, Lahti Aqua Ltd, and Lahti Energy Ltd.
Author information
Authors and Affiliations
Contributions
JK and IM: were responsible for developing and running the monitoring of Lake Vesijärvi. KS and KV: made data analysis. KS: wrote the manuscript together with KV and MK, and all authors contributed to reviewing and editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Luigi Naselli-Flores
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salonen, K., Vuorio, K., Ketola, M. et al. Development of phytoplankton of Lake Vesijärvi during recovery from eutrophication. Hydrobiologia 850, 947–966 (2023). https://doi.org/10.1007/s10750-022-05136-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-05136-9