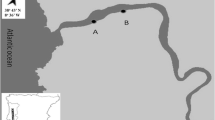
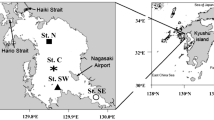
Abstract
Estuarine intertidal flats are important ecosystems characterized by high primary production of microphytobenthos and high secondary production of macro- and meiofauna, especially nematodes. However, the link between both ecosystem components (microphytobenthos and faunal communities) is not fully established yet. In this study, spatial patterns and drivers of nematode density and genus composition were investigated at two different spatial scales (i.e. meso- and microscale), with drivers including sediment granulometry, inundation period and food availability as indicated by various phytopigments. Our study has shown that specific food sources, as represented by different pigments and measures of freshness, are important drivers of nematode genus composition and densities at both scales, especially for the surface layers of the sediments. These food sources mainly comprise microphytobenthos, but also deposited phytodetritus and zooplankton faecal pellets, a resource which had hitherto been largely overlooked in intertidal flats. Tidal level and grain size also had a more pronounced structuring effect in the surface layer of the sediment, while their assumed larger importance at the mesoscale was not outspoken. At both scales, vertical heterogeneity in nematode assemblages was larger than horizontal variability, which has repercussions for future studies into the spatial variability of nematode assemblages of tidal flats.







Similar content being viewed by others
References
Anderson, M. J. & J. Robinson, 2003. Generalized discriminant analysis based on distances. Australian & New Zealand Journal of Statistics 45: 301–318. https://doi.org/10.1111/1467-842X.00285.
Anderson, M. J., R. N. Gorley & K. R. Clarke, 2008. PERMANOVA + for PRIMER: Guide to Software and Statistical Methods. PRIMER-E Ltd, Plymouth.
Armonies, W. & K. Reise, 2000. Faunal diversity across a sandy shore. Marine Ecology Progress Series 196: 49–57. https://doi.org/10.3354/meps196049.
Bezerra, T. et al., 2018. NeMys: world database of free-living marine nematodes
Blanchard, G., 1990. Overlapping microscale dispersion patterns of meiofauna and microphytobenthos. Marine Ecology Progress Series 68: 101–111.
Blome, D., U. Schleier & K. H. von Bernem, 1999. Analysis of the small-scale spatial patterns of free-living marine nematodes from tidal flats in the East Frisian Wadden Sea. Marine Biology 133: 717–726. https://doi.org/10.1007/s002270050513.
Boldina, I., P. G. Beninger & M. Le Coz, 2014. Effect of long-term mechanical perturbation on intertidal soft-bottom meiofaunal community spatial structure. Journal of Sea Research 85: 85–91. https://doi.org/10.1016/j.seares.2013.10.006.
Boon, A. & G. Duineveld, 1996. Phytopigments and fatty acids as molecular markers for the quality of near-bottom particulate organic matter in the North Sea. Journal of Sea Research 35: 279–291. https://doi.org/10.1016/S1385-1101(96)90755-8.
Boucher, G., 1990. Pattern of nematode species diversity in temperate and tropical subtidal sediments. Marine Ecology 11: 133–146. https://doi.org/10.1111/j.1439-0485.1990.tb00234.x.
Braeckman, U., P. Provoost, T. Moens, K. Soetaert, J. J. Middelburg, M. Vincx & J. Vanaverbeke, 2011a. Biological vs. physical mixing effects on benthic food web dynamics. PLoS ONE 6: e18078. https://doi.org/10.1371/journal.pone.0018078.
Braeckman, U., C. Van Colen, K. Soetaert, M. Vincx & J. Vanaverbeke, 2011b. Contrasting macrobenthic activities differentially affect nematode density and diversity in a shallow subtidal marine sediment. Marine Ecology Progress Series 422: 179–191. https://doi.org/10.3354/meps08910.
Carpentier, A., S. Como, C. Dupuy, C. Lefrancois & E. Feunteun, 2014. Feeding ecology of Liza spp. in a tidal flat: evidence of the importance of primary production (biofilm) and associated meiofauna. Journal of Sea Research 92: 86–91. https://doi.org/10.1016/j.seares.2013.10.007.
Cibic, T., O. Blasutto & N. Bettoso, 2009. Microalgal–meiofaunal interactions in a sublittoral site of the Gulf of Trieste (northern Adriatic Sea, Italy): a three-year study. Journal of Experimental Marine Biology and Ecology 370: 144–154. https://doi.org/10.1016/j.jembe.2008.12.006.
Commito, J. A. & G. Tita, 2002. Differential dispersal rates in an intertidal meiofauna assemblage. Journal of Experimental Marine Biology and Ecology 268: 237–256. https://doi.org/10.1016/S0022-0981(01)00386-0.
De Grisse, A., 1965. A labour-saving method for fixing and transferring eelworms to anhydrous glycerin. Landbouw Hogeschool, OpzoekStns—Leerstoel Dierkunde, Gent
D’Hondt, A.-S., W. Stock, L. Blommaert, T. Moens & K. Sabbe, 2018. Nematodes stimulate biomass production in a multispecies diatom biofilm. Marine Environmental Research 140: 78–89. https://doi.org/10.1016/j.marenvres.2018.06005.
Ferreira, R. C., A. B. Nascimento, P. J. P. Santos, M. L. Botter-Carvalho & T. K. Pinto, 2015. Responses of estuarine nematodes to an increase in nutrient supply: an in situ continuous addition experiment. Marine Pollution Bulletin 90: 115–120. https://doi.org/10.1016/j.marpolbul.2014.11.012.
Fonseca, G., P. Hutchings, D. C. Vieira & F. Gallucci, 2011. Meiobenthic community underneath the carcass of a stingray: a snapshot after natural death. Aquatic Biology 13: 27–33. https://doi.org/10.3354/ab00347.
Franco, M. A., K. Soetaert, M. J. Costa, M. Vincx & J. Vanaverbeke, 2008. Uptake of phytodetritus by meiobenthos using C13 labelled diatoms and Phaeocystis in two contrasting sediments from the North Sea. Journal of Experimental Marine Biology and Ecology 362: 1–8. https://doi.org/10.1016/j.jembe.2008.04.010.
Gallucci, F., M. Steyaert & T. Moens, 2005. Can field distributions of marine predacious nematodes be explained by sediment constraints on their foraging success? Marine Ecology Progress Series 304: 167–178. https://doi.org/10.3354/meps304167.
Gallucci, F., G. Fonseca & M. Brustolin, 2019. Hydrodynamic exposure decreases the role of environmental filtering over benthic coastal metacommunities. In Adão, H., C. Vicente, K. Sroczyńska, M. Espada, P. Alvim, M. Costa & S. Vieira (eds), Book of Abstracts, SeventIMCO—Seventeenth International Meiofauna Conference, Portugal, University of Évora: 36
Gheskiere, T., E. Hoste, J. Vanaverbeke, M. Vincx & S. Degraer, 2004. Horizontal zonation patterns and feeding structure of marine nematode assemblages on a macrotidal, ultra-dissipative sandy beach (De Panne, Belgium). Journal of Sea Research 52: 211–226. https://doi.org/10.1016/j.seares.2004.02.001.
Giere, O., 2009. Meiobenthology: The Microscopic Motile Fauna of Aquatic Sediments. Springer, Berlin.
Gingold, R., M. Mundo-Ocampo, O. Holovachov & A. Rocha-Olivares, 2010. The role of habitat heterogeneity in structuring the community of intertidal free-living marine nematodes. Marine Biology 157: 1741–1753. https://doi.org/10.1007/s00227-010-1447-z.
Gingold, R., S. E. Ibarra-Obando & A. Rocha-Olivares, 2011. Spatial aggregation patterns of free-living marine nematodes in contrasting sandy beach micro-habitats. Journal of the Marine Biological Association of the United Kingdom 91: 615–622. https://doi.org/10.1017/S0025315410001128.
Heip, C., M. Vincx & G. Vranken, 1985. The ecology of marine nematodes. Oceanography and Marine Biology: An Annual Review 23: 399–489.
Heip, C. H. R., N. K. Goosen, P. M. J. Herman, J. Kromkamp, J. J. Middelburg & J. Soetaert, 1995. Production and consumption of biological particles in temperate tidal estuaries. Oceanography and Marine Biology: an Annual Review 33: 1–149.
Herman, P., J. Middelburg, J. Van de Koppel & C. Heip, 1999. Ecology of estuarine macrobenthos. Advances in Ecological Research 29: 195–240.
Herman, P. M., J. J. Middelburg & C. H. Heip, 2001. Benthic community structure and sediment processes on an intertidal flat: results from the ECOFLAT project. Continental Shelf Research 21: 2055–2071. https://doi.org/10.1016/S0278-4343(01)00042-5.
Higgins, R. P. & H. Thiel, 1988. Introduction to the Study of Meiofauna. Smithsonian Institution Press, Washington, DC: 488.
Hodda, M., 1990. Variation in estuarine littoral nematode populations over three spatial scales. Estuarine, Coastal and Shelf Science 30: 325–340. https://doi.org/10.1016/0272-7714(90)90001-8.
Hooper, D. U., et al., 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs 75: 3–35. https://doi.org/10.1890/04-0922.
Hubas, C., C. Sachidhanandam, H. Rybarczyk, H. V. Lubarsky, A. Rigaux, T. Moens & D. M. Paterson, 2010. Bacterivorous nematodes stimulate microbial growth and exopolymer production in marine sediment microcosms. Marine Ecology Progress Series 419: 85–94. https://doi.org/10.3354/meps08851.
Joint, I. R., J. M. Gee & R. M. Warwick, 1982. Determination of fine-scale vertical-distribution of microbes and meiofauna in an intertidal sediment. Marine Biology 72: 157–164. https://doi.org/10.1007/BF00396916.
Kromkamp, J. C., J. F. C. de Brouwer, G. F. Blanchard, R. M. Forster & V. Creach, 2006. Functioning of Microphytobenthos in Estuaries: Proceedings of the Colloquium, Amsterdam, 21–23 August 2003. Royal Netherlands Academy of Arts and Sciences, Amsterdam
Le Hir, P., W. Roberts, O. Cazaillet, M. Christie, P. Bassoullet & C. Bacher, 2000. Characterization of intertidal flat hydrodynamics. Continental Shelf Research 20: 1433–1459. https://doi.org/10.1016/S0278-4343(00)00031-5.
Lucas, C. H., J. Widdows, M. D. Brinsley, P. N. Salkeld & P. M. J. Herman, 2000. Benthic-pelagic exchange of microalgaeat a tidal flat. 1. Pigment analysis. Marine Ecology Progress Series 196: 59–73. https://doi.org/10.3354/meps196059.
MacIntyre, H. L., R. J. Geider & D. C. Miller, 1996. Microphytobenthos: the ecological role of the “secret garden” of unvegetated, shallow-water marine habitats. I. Distribution, abundance and primary production. Estuaries 19: 186–201. https://doi.org/10.2307/1352224.
Maria, T. F., J. Vanaverbeke, R. Gingold, A. M. Esteves & A. Vanreusel, 2013. Tidal exposure or microhabitats: what determines sandy-beach nematode zonation? a case study of a macrotidal ridge-and-runnel sandy beach in Belgium. Marine Ecology 34: 207–217. https://doi.org/10.1111/maec.12008.
McLachlan, A. & E. Jaramillo, 1996. Zonation on sandy beaches. Oceanographic Literature Review 12: 1247.
Meire, P., T. Ysebaert, S. V. Damme, E Vd Bergh, T. Maris & E. Struyf, 2005. The Scheldt estuary: a description of a changing ecosystem. Hydrobiologia 540: 1–11. https://doi.org/10.1007/s10750-005-0896-8.
Middelburg, J. J., C. Barranguet, H. T. S. Boschker, P. M. J. Herman, T. Moens & C. H. R. Heip, 2000. The fate of intertidal microphytobenthos carbon: an in situ C13-labeling study. Limnology and Oceanography 45(6): 1224–1234. https://doi.org/10.4319/lo.2000.45.6.1224.
Moens, T. & P. G. Beninger, 2018. Meiofauna: an inconspicuous but important player in mudflat ecology. In Beninger, P. G. (ed.), Mudflat Ecology. Springer, Berlin: 91–148.
Moens, T. & M. Vincx, 1997. Observations on the feeding ecology of estuarine nematodes. Journal of the Marine Biological Association of the United Kingdom 77: 211–227. https://doi.org/10.1017/S0025315400033889.
Moens, T. & M. Vincx, 2000. Temperature, salinity and food thresholds in two brackish-water bacterivorous nematode species: assessing niches from food absorption and respiration experiments. Journal of Experimental Marine Biology and Ecology 243: 137–154. https://doi.org/10.1016/S0022-0981(99)00114-8.
Moens, T., D. Van Gansbeke & M. Vincx, 1999. Linking estuarine nematodes to their suspected food. A case study from the Westerschelde Estuary (south-west Netherlands). Journal of the Marine Biological Association of the United Kingdom 79: 1017–1027. https://doi.org/10.1017/s0025315499001253.
Moens, T., P. Herman, L. Verbeeck, M. Steyaert & M. Vincx, 2000. Predation rates and prey selectivity in two predacious estuarine nematode species. Marine Ecology Progress Series 205: 185–193. https://doi.org/10.3354/meps205185.
Moens, T., C. Luyten, J. J. Middelburg, P. M. J. Herman & M. Vincx, 2002. Tracing organic matter sources of estuarine tidal flat nematodes with stable carbon isotopes. Marine Ecology Progress Series 234: 127–137. https://doi.org/10.3354/meps234127.
Moens, T., S. Bouillon & F. Gallucci, 2005. Dual stable isotope abundances unravel trophic position of estuarine nematodes. Journal of the Marine Biological Association of the United Kingdom 85: 1401–1407. https://doi.org/10.1017/S0025315405012580.
Moens, T., S. Vanhove, I. De Mesel, B. Kelemen, T. Janssens, A. Dewicke & A. Vanreusel, 2007. Carbon sources of Antarctic nematodes as revealed by natural carbon isotope ratios and a pulse-chase experiment. Polar Biology 31: 1–13. https://doi.org/10.1007/s00300-007-0323-x.
Moens, T., et al., 2013. Ecology of free-living marine nematodes. In Schmidt-Rhaesa, A. (ed.), Handbook of Zoology. De Gruyter, Berlin: 109–152.
Moens, T., A.-M. Vafeiadou, E. De Geyter, P. Vanormelingen, K. Sabbe & M. De Troch, 2014. Diatom feeding across trophic guilds in tidal flat nematodes, and the importance of diatom cell size. Journal of Sea Research 92: 125–133. https://doi.org/10.1016/j.seares.2013.08.007.
Montagna, P. A., B. C. Coull, T. L. Herring & B. W. Dudley, 1983. The relationship between abundances of meiofauna and their suspected microbial food (diatoms and bacteria). Estuarine, Coastal and Shelf Science 17: 381–394. https://doi.org/10.1016/0272-7714(83)90124-5.
Netto, S. A. & A. Meneghel, 2014. Pulse of marine subsidies: the role of surf diatom Asterionellopsis glacialis accumulations in structuring the meiofauna of sandy beaches. Marine Biodiversity 44: 445–457. https://doi.org/10.1007/s12526-014-0253-0.
Nicholas, W. L. & M. Hodda, 1999. The free-living nematodes of a temperate, high energy, sandy beach: faunal composition and variation over space and time. Hydrobiologia 394: 113–127. https://doi.org/10.1023/a:1003544115600.
Peters, K., C. Walters & J. Moldowan, 2005. The Biomarker Guide. Cambridge University Press, Cambridge, UK.
Pinckney, J. & R. Sandulli, 1990. Spatial autocorrelation analysis of meiofaunal and microalgal populations on an intertidal sandflat: scale linkage between consumers and resources. Estuarine, Coastal and Shelf Science 30: 341–353. https://doi.org/10.1016/0272-7714(90)90002-9.
Pinckney, J. L., K. R. Carman, S. E. Lumsden & S. N. Hymel, 2003. Microalgal-meiofaunal trophic relationships in muddy intertidal estuarine sediments. Aquatic Microbial Ecology 31: 99–108. https://doi.org/10.3354/ame031099.
Platt, H. M. & R. M. Warwick, 1983. A Synopsis of the Freeliving Marine Nematodes. Part 1: British Enoplids. Cambridge University Press, Cambridge.
R Core Team, 2013. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Viennva, Austria. http://www.R-project.org/
Rzeznik Orignac, J., G. Boucher, D. Fichet & P. Richard, 2008. Stable isotope analysis position of intertidal of food source and trophic nematodes and copepods. Marine Ecology Progress Series 359: 145–150. https://doi.org/10.3354/meps07328.
Sahraean, N., T. C. Bezerra, K. E. Khanaghah, H. Mosallanejad, E. Van Ranst & T. Moens, 2017. Effects of pollution on nematode assemblage structure and diversity on beaches of the northern Persian Gulf. Hydrobiologia 799: 349–369. https://doi.org/10.1007/s10750-017-3234-z.
Saidi, I., N. Essid, F. Boufahja, A. Nasri, A. Hannachi, B. Nefzi & H. Beyrem, 2017. The effects of raw effluents from pulp and paper industry from Tunisia on marine nematodes: a microcosm bioassay. Cahiers De Biologie Marine 58: 387–395. https://doi.org/10.21411/cbm.a.c942a02d.
Schratzberger, & J. Ingels, 2018. Meiofauna matters: the roles of meiofauna in benthic ecosystems. Journal of Experimental Marine Biology and Ecology 502: 12–25. https://doi.org/10.1016/j.jembe.2017.01.007.
Seinhorst, J., 1959. A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica 4: 67–69.
Semprucci, F., P. Colantoni, G. Baldelli, M. Rocchi & M. Balsamo, 2010. The distribution of meiofauna on back-reef sandy platforms in the Maldives (Indian Ocean). Marine Ecology-an Evolutionary Perspective 31: 592–607. https://doi.org/10.1111/j.1439-0485.2010.00383.x.
Semprucci, F., M. Balsamo & R. Sandulli, 2016. Assessment of the ecological quality (EcoQ) of the Venice lagoon using the structure and biodiversity of the meiofaunal assemblages. Ecological Indicators 67: 451–457. https://doi.org/10.1016/j.ecolind.2016.03.014.
Somerfield, P. J., S. L. Dashfield & R. M. Warwick, 2007. Three-dimensional spatial structure: nematodes in a sandy tidal flat. Marine Ecology Progress Series 336: 177–186. https://doi.org/10.3354/meps336177.
Steyaert, M., N. Garner, D. van Gansbeke & M. Vincx, 1999. Nematode communities from the North Sea: environmental controls on species diversity and vertical distribution within the sediment. Journal of the Marine Biological Association of the United Kingdom 79: 253–264. https://doi.org/10.1017/S0025315498000289.
Steyaert, M., P. M. J. Herman, T. Moens, J. Widdows & M. Vincx, 2001. Tidal migration of nematodes on an estuarine tidal flat (the Molenplaat, Schelde Estuary, SW Netherlands). Marine Ecology Progress Series 224: 299–304. https://doi.org/10.3354/meps224299.
Steyaert, M., J. Vanaverbeke, A. Vanreusel, C. Barranguet, C. Lucas & M. Vincx, 2003. The importance of fine-scale, vertical profiles in characterising nematode community structure. Estuarine, Coastal and Shelf Science 58: 353–366. https://doi.org/10.1016/S0272-7714(03)00086-6.
Thomas, M. C. & P. C. Lana, 2011. A new look into the small-scale dispersal of free-living marine nematodes. Zoologia 28: 449–456. https://doi.org/10.1590/S1984-46702011000400006.
Underwood, G. & J. Kromkamp, 1999. Primary production by phytoplankton and microphytobenthos in estuaries. Advances in Ecological Research 29: 93–153.
Underwood, A., M. Chapman & S. Connell, 2000. Observations in ecology: you can’t make progress on processes without understanding the patterns. Journal of Experimental Marine Biology and Ecology 250: 97–115. https://doi.org/10.1016/S0022-0981(00)00187-7.
Urban-Malinga, B. & T. Moens, 2006. Fate of organic matter in Arctic intertidal sediments: is utilisation by meiofauna important? Journal of Sea Research 56: 239–248. https://doi.org/10.1016/j.seares.2006.05.003.
Urkmez, D., M. L. Brennan, M. Sezgin & L. Bat, 2015. A brief look at the free-living Nematoda of the oxic/anoxic interface with a new genus record (Trefusia) for the Black Sea. Oceanological and Hydrobiological Studies 44: 539–551. https://doi.org/10.1515/ohs-2015-0051.
Vafeiadou, A.-M., P. Materatski, H. Adão, M. De Troch & T. Moens, 2014. Resource utilization and trophic position of nematodes and harpacticoid copepods in and adjacent to Zostera noltii beds. Biogeosciences 11: 4001–4014. https://doi.org/10.5194/bg-11-4001-2014.
Vanaverbeke, J., T. Gheskiere, M. Steyaert & M. Vincx, 2002. Nematode assemblages from subtidal sandbanks in the Southern Bight of the North Sea: effect of small sedimentological differences. Journal of Sea Research 48: 197–207. https://doi.org/10.1016/S1385-1101(02)00165-X.
Vanaverbeke, J., M. Franco, D. van Oevelen, L. Moodley, P. Provoost, et al., 2008. Benthic responses to sedimentation of phytoplankton on the Belgian Continental Shelf. In Rousseau, V., C. Lancelot & D. Cox (eds), Current Status of Eutrophication in the Belgian Coastal Zone. Presses Universitaires de Bruxelles, Brussels: 73–90.
Vieira, D. C. & G. Fonseca, 2013. The importance of vertical and horizontal dimensions of the sediment matrix in structuring nematodes across spatial scales. PLoS ONE 8: e77704. https://doi.org/10.1371/journal.pone.0077704.
Warwick, R. M., H. M. Platt & P. J. Somerfield, 1998. Freeliving marine nematodes: part III. Monhysterida. Synopses of the British Fauna No. 53. Field Studies Council, Shrewsbury
Wentworth, C. K., 1922. A scale of grade and class terms for clastic sediments. The Journal of Geology 30: 377–392.
Wright, S. & S. Jeffrey, 1997. High-resolution HPLC system for chlorophylls and carotenoids of marine phytoplankton. In Jeffrey, S. W., R. F. C. Mantoura & S. W. Wright (eds), Phytoplankton Pigments in Oceanography: Guidelines to Moder Methods. UNESCO, Paris.
Acknowledgements
Field samples from the microscale stations were collected in collaboration with NIOZ, which provided the necessary permit for field sampling, issued by the Province of Zeeland, The Netherlands, “Directie Ruimte, Milieu en Water.” Annick Van Kenhove and Guy De Smet provided invaluable support with making slides of nematodes. Dirk Van Gansbeke performed the pigment analyses and Bart Beuselinck completed the sediment granulometry analyses and total organic matter measurements. Niels Viaene is acknowledged for help during field sampling and extraction of nematodes. Renata Mamede da Silva Alves is acknowledged for making sampling maps. Two anonymous reviewers provided valuable feedback that helped to improve the manuscript.
Funding
The first author received a Ph.D. Grant of the Chinese Scholarship Council (2011633060) from November 2011 to November 2015, and received further financial support from the Flemish Science Fund FWO (G0H3817N). Additional support was provided by the special research fund of Ghent University (BOF 01SC3312) from March 2012 to October 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed by the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements, if applicable.
Additional information
Handling editor: Pierluigi Viaroli
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, X., Vanreusel, A., Hauquier, F. et al. Environmental drivers of nematode abundance and genus composition at two spatial scales on an estuarine intertidal flat. Hydrobiologia 846, 193–214 (2019). https://doi.org/10.1007/s10750-019-04064-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-04064-5