Abstract
We examined how freshwater flow and phytoplankton biomass affected abundance and population dynamics of the introduced subtropical copepod Pseudodiaptomus forbesi in brackish and freshwater regions of the San Francisco Estuary, California, USA. This copepod is key prey for the endangered and food-limited delta smelt, Hypomesus transpacificus, in low-salinity water during summer–autumn. Long-term monitoring data showed that P. forbesi was most abundant in fresh water, where summer–autumn abundance was invariant with freshwater flow. Abundance was positively related to freshwater flow in low-salinity water. Reproductive rates in both regions during 2010–2012 were low and unresponsive to chlorophyll or freshwater flow. Development indices, calculated as ratios of laboratory-derived to field-derived stage durations, were lowest for nauplii and highest for late copepodites, but averaged below 0.5 for all stages combined. Development indices were weakly related to chlorophyll for late copepodites only, unrelated to freshwater flow, and slightly higher in low-salinity than fresh water. Thus, the principal mechanism by which flow affects the P. forbesi population is apparently transport of copepods from fresh water to low-salinity water, where copepods are available to delta smelt. This work demonstrates how freshwater flow affects estuarine foodwebs through spatial subsidies of food supply.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater flow is a dominant influence on the state of estuaries. It can be the principal driver of interannual and seasonal variability in distributions of salinity and therefore biota, and can influence productivity at all trophic levels (Skreslet, 1986). Climate change and increasing demand are expected to reduce the availability of fresh water to many estuaries, altering the magnitude and timing of fluctuations in flow and in these responses. Therefore, we need to understand better how variation in freshwater flow in estuaries influences physical properties and biological responses.
Several impediments limit progress in understanding the mechanisms underlying biotic responses to freshwater flow. Numerous mechanisms potentially contribute to these responses, such as variation in nutrient loading, stratification, and predator-prey interactions (Drinkwater & Frank, 1994; Alber, 2002; Kimmerer, 2002), and these may operate at different seasons and locations. At the landscape scale, the geomorphic, hydrologic, and biological complexity of most estuaries adds variability that may interfere with detection of mechanisms of change that are related to flow. For pelagic organisms which live in a moving frame of reference (Laprise & Dodson, 1993), flow effects are probably best analyzed in a Lagrangian or moving frame of reference, which can be difficult in stratified estuaries and where mechanisms for flow effects have a geomorphic component.
To overcome these impediments and determine mechanisms for flow effects on pelagic biota, investigations must determine how demographic processes of birth, development, mortality, and movement respond to flow and other environmental influences. For example, in a population that increases with increasing freshwater flow, a positive relationship of birth or growth rate to flow would suggest a mechanism related to food supply. Similarly, a negative relationship of mortality to flow might suggest that predation was reduced by high flow. Only by understanding how flow affects these processes, it is possible to interpret how abundance patterns vary with flow.
In the San Francisco Estuary (SFE), annual abundance indices of several species of fish and one macroinvertebrate vary with freshwater flow (Jassby et al., 1995). This variation appears to be a result of direct mechanisms rather than trophic effects because abundance of their zooplankton prey does not appear to vary with freshwater flow (Kimmerer, 2002; Kimmerer et al., 2013). However, abundance alone is an incomplete measure of productivity, and little is known about how population dynamics of estuarine zooplankton responds to flow or to phytoplankton biomass, which may itself respond to flow (Drinkwater & Frank, 1994).
This paper examines the abundance, egg production rate, development, and growth of the introduced calanoid copepod Pseudodiaptomus forbesi to variation in freshwater flow and phytoplankton biomass in the brackish to freshwater region of the San Francisco Estuary. This species is most abundant during summer–autumn, and is more abundant in fresh water than in the low-salinity zone (0.5–5 salinity) where it is an important prey item for the endangered delta smelt, Hypomesus transpacificus. Our objective was to understand the role that variable freshwater flow plays in the population dynamics of P. forbesi and, in particular, how demography and transport interact to influence the availability of food for fishes.
Methods
Our study combined data from long-term monitoring programs on copepod abundance with short-term studies conducted in 2010–2012. These 3 years provided a contrast in flow, with 2010 and 2012 being dry and 2011 wet, and in phytoplankton biomass, with a spike in chlorophyll concentration in autumn of 2012. The short-term studies included transects for spatial patterns of abundance by life stage and reproductive rate and molt-rate experiments to determine stage durations and growth rates. A laboratory experiment with excess food was conducted to provide the upper limit of development rate by which to assess the degree of food limitation in the field.
Study site and species
The San Francisco Estuary (SFE) includes San Francisco and San Pablo bays (not shown), Suisun Bay, and the Delta at the confluence of the Sacramento and San Joaquin Rivers in California’s Central Valley (Fig. 1). The SFE is mesotidal, turbid, and rather unproductive (Cloern & Jassby, 2012). The climate is Mediterranean, with a warm dry season from June through October and a cool wet season from November through May. Interannual variability in flow is high: mean flows by water year (October–September) during 1956–2015 ranged from 97 to 2526 m3 s−1 (1976–1977 and 1982–1983, respectively), a 26-fold range (see below for data source).
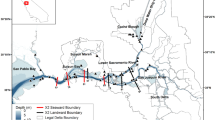
Map and diagram of study area with inset showing location within California, USA. Circles and crosses, transect stations, 12 stations each on the Sacramento and San Joaquin rivers at intervals of ~ 3 km; triangles, stations in long-term monitoring program; white triangles, fresh water (salinity < 0.5) and black triangles, low-salinity (salinity 0–0.5–5)
Our focus is on the dry season in the northern, river-dominated part of the estuary including the broad, shallow Suisun Bay and the Delta, a patchwork of islands largely devoted to agriculture and separated by a network of tidal sloughs and channels (Fig. 1). Freshwater flow into the Delta is controlled by releases from reservoirs except when runoff is very high, usually in January–April. During the dry season, water is released to flow down the Sacramento River to the Delta where up to half of the flow is diverted by pumping from tidal fresh water in the southern Delta (Fig. 1) to be delivered to farms and cities to the south. During summers, the contribution of the San Joaquin and other smaller rivers to estuarine flow is usually negligible.
The modification of flows and diversion of fresh water have led to conflicts over environmental effects and particularly over protection of fishes that are affected by these practices, notably salmon and delta smelt, which has declined in abundance over the last ~ two decades and is now considered at imminent risk of extinction (Moyle et al., 2016). However, the decline in delta smelt is due at least in part to food limitation at various life stages (Bennett, 2005; Slater & Baxter, 2014; Hammock et al., 2015). Primary productivity and abundance of the copepod prey of this fish have been low since ~ 1987 mainly because of grazing by the clam Potamocorbula amurensis, introduced in 1986 (Cloern & Jassby, 2012). A spate of copepod introductions during 1979–1993 further altered the species composition of the zooplankton and thereby the feeding environment of delta smelt (Orsi & Walter, 1991; Orsi & Ohtsuka, 1999).
Pseudodiaptomus forbesi was introduced from Asia to the SFE in 1987 (Orsi & Walter, 1991), and soon became the numerically dominant calanoid copepod in the northern reaches of the estuary. After its introduction, P. forbesi was most abundant in waters ranging in salinity from 0 to 16 (Orsi & Walter, 1991), although it is now limited to salinity below ~ 5 (Kayfetz & Kimmerer, 2017). P. forbesi is an important food source for larvae and juveniles of pelagic fishes (Meng & Orsi, 1991; Moyle & Leidy, 1992; Bryant & Arnold, 2007) and makes up over half of the summer–autumn diet of delta smelt (Nobriga, 2002; Slater & Baxter, 2014). P. forbesi is a suspension-feeder, consuming phytoplankton, and microzooplankton (Bouley & Kimmerer, 2006; York et al., 2014; Bowen et al., 2015).
Long-term monitoring data
Data on freshwater flow were obtained from a program that uses a daily volume balance to calculate flow into the estuary (net Delta outflow, http://www.water.ca.gov/dayflow/). Daily water temperature was calculated as the median of hourly temperature data from a continuous monitoring station at Antioch, California (Fig. 1; http://cdec.water.ca.gov/for station ANH). Daily solar radiation was obtained from the California Irrigation Management Information System (http://wwwcimis.water.ca.gov) for two monitoring sites near the Delta.
The California Interagency Ecological Program has run a zooplankton monitoring program since 1972 (Orsi & Mecum, 1986), from which we used data from summer to autumn of 1994–2015, after the last of the copepod introductions (Orsi & Ohtsuka, 1999). During this period, samples have been taken monthly at a median of 17 stations throughout the Delta and Suisun and San Pablo bays (Fig. 1). Samples were collected with a 10-cm diameter Clarke-Bumpus net with a flowmeter, towed for 10 min obliquely from near bottom to the surface. Counts of organisms in subsamples were used to calculate abundance m−3 by species and gross life stage (Orsi & Mecum,1986).
Data from three stations in the central Delta (Fig. 1) were used to represent the freshwater population center of P. forbesi; these stations have complete records for the study period and are unaffected by salinity intrusion from the west. Data from the low-salinity zone (LSZ, 0.5–5 salinity) were used to represent the habitat of delta smelt. This set of stations excluded two stations in Suisun Marsh (Fig. 1), which is somewhat isolated from Suisun Bay, and stations in the Delta where salinity can be elevated by agricultural runoff rather than the ocean source. We examined the seasonal pattern of abundance of total copepodites and adults in the freshwater population center and the responses of abundance in the freshwater region and the LSZ to freshwater flow. To represent the timing of the spring increase, we determined the Julian day on which the linearly interpolated abundance reached 1000 m−3.
Laboratory-based mass, stage duration, and growth
Copepodites and adults for mass estimates were collected with a 53-µm mesh, 0.5-m diameter plankton net on 20 July 2009 from the upper estuary (Fig. 1) at a temperature of 24°C and salinity of 0.5. Plankton were preserved in 5% glutaraldehyde (Kimmerer & McKinnon, 1987) and held for 5 months. Copepods were then rinsed and sorted and 30–50 of each life stage were transferred into separate pre-weighed (Sartorius SE-2 microbalance) tin capsules. Samples were dried at 60°C for 2 days and weighed again, then analyzed for carbon and nitrogen content using a Costech ECS 4010 elemental analyzer calibrated with the standard Cystine OAS (B2105, Elemental Microanalysis). Egg carbon was estimated from measured diameter (Kimmerer et al., 2014b).
Two experiments were run to determine durations of all stages. Methods generally followed those used previously for the co-occurring cyclopoid Limnoithona tetraspina (Gould & Kimmerer, 2010; Kimmerer & Gould, 2010). Pseudodiaptomus forbesi was collected on 20 July 2012 from the upper estuary (Fig. 1) by gentle, subsurface horizontal tows with a 300-µm mesh, 0.5-m diameter plankton net; surface water temperature was 20.7°C and surface salinity 0.6. Copepods were immediately placed into 20-L acid-washed insulated buckets containing in situ water and transported to the Romberg Tiburon Center (~ 40 km southwest of Suisun Bay, Fig. 1). Water from the collection site was filtered through a 35-µm mesh sieve to remove mesozooplankton.
In the laboratory, ~ 800 ovigerous female P. forbesi were isolated from the sample and transferred into a 20-cm, 200-µm mesh sieve suspended in a 10-L bucket containing 35-µm filtered in situ water. Female copepods were held for 6 h within the sieve, then successively transferred and held for 6 h in two other buckets, providing three cohorts called Experiment 1. Because the number of nauplii per sample was lower than expected, we sorted and started another set of cohorts a day after the first set (Experiment 2). Note that this procedure did not provide true replicates.
Incubation buckets were placed in a water bath set to 22 ± 0.1°C and the water was aerated lightly. Approximately 1/3 of the water was changed every 3 days using freshly collected 35-µm filtered in situ water. Copepods were fed daily at saturation (Experiment 1: mean 1520 µg C L−1, range 1397–1695 µg C L−1; Experiment 2: mean 1334 µg C L−1, range 939–1789 µg C L−1) with a mixture of roughly equal biomass of Cryptomonas ovata (UTEX LB 358), Chlamydomonas reinhardtii (UTEX 90), Selenastrum capricornutum (UTEX 1648), Scenedesmus obliquus (UTEX 393), and cryopreserved phytoplankton (Shellfish Diet®, Reed Mariculture Inc.). The phytoplankton species were selected based on preliminary work to determine what food sources would support rapid growth through all life stages.
Samples for particulate organic carbon content were collected daily from each incubation bucket; 100 mL of water was filtered onto pre-combusted 25-mm diameter Whatman GF/F filters. The filters were dried at 60°C for at least 2 days, rolled in tin, and pressed into pellets. Carbon and nitrogen content were determined by the UC Davis Stable Isotope Facility.
Each incubation bucket was sampled twice a day at approximately 0800 and 1800. The contents of each bucket were mixed, and a 200-mL sample was taken with a beaker, concentrated on a 35-µm mesh sieve, transferred to a 20-mL scintillation vial, stained with the vital stain Neutral Red, and preserved with 4% (final concentration) buffered formaldehyde. Copepods that had taken up the stain (99% of all copepods) were later identified to stage and counted under a dissecting microscope. Mean counts per sample were 54 in Experiment 1 and 68 in Experiment 2.
As expected from the low initial abundance of nauplii, Experiment 1 lacked enough individuals to maintain the initial sampling rate through all life stages, so we used Experiment 1 to determine stage durations of nauplii and preliminary estimates of duration of copepodites. These were updated using data from Experiment 2, in which we began sampling 2 days after initiation when the cohorts had reached nauplius stage 5 (N5). In Experiment 1, some females went through the 200-µm mesh sieve and produced additional clutches, resulting in a bimodal distribution by life stage in some samples. We corrected for this distortion (see online Appendix), but even without correction, the distortion affected results only for copepodites, whose stage durations were determined mainly from Experiment 2 as discussed below.
Field sampling
During August–October 2010–2012, we conducted two transects per year up the Sacramento River and two up the San Joaquin River to sample for zooplankton (Table 1). Transects began at the western margin of the Delta and twelve samples were taken at ~ 3 km intervals up each river (Fig. 1). Samples were taken at each station by vertical tows of a 0.5-m diameter, 53-µm net equipped with a flowmeter, and surface salinity and temperature were determined using a SeaBird SBE-19 CTD or a YSI Model 30. Although sampling plankton according to salinity is generally preferable to geographic-based sampling in an estuary (Laprise & Dodson, 1993; Kimmerer et al., 2014b), in this study, we sampled geographically (because much of our sampling was in fresh water) and later stratified the data by salinity.
Subsamples were taken from a known volume with a piston pipette and counted under a dissecting microscope. We counted at least 500 total copepods of all stages, except above station 7 on the Sacramento River (Fig. 1) where copepods were uncommon. Copepodites were identified to stage, and adults and stage 5 copepodites (C5) to sex. Eggs were counted in sacs carried by females and in loose egg sacs identifiable as those of P. forbesi. Eggs were not counted in one pair of transects in 2010. Egg ratios were calculated as total eggs divided by total females, and egg production rate was determined by the egg ratio method of Edmondson et al., (1962) using egg durations from Sullivan & Kimmerer, (2013). No correction for female mortality was applied because of practical difficulties (Ohman et al., 1996; Kimmerer, 2015).
Molt-rate experiments
Experiments using the Modified Molt-Rate method (Hirst et al., 2014) were conducted within 2 days of each transect using copepods collected from salinities of 0.5 and 2 along the transect lines. Nauplii were collected with a 53-µm net and copepodites with a 150-µm net, both towed gently just below the surface for ~ 5 min and handled as for development experiments.
In the laboratory, individual copepods were sorted under dissecting microscopes to obtain nauplii and early and late copepodites. A target of 30 copepods per sample was achieved with the exception of a few experiments because of low catches in the field. To minimize handling time, life stages were estimated rapidly and actual stages confirmed after incubation. Nauplii were 56% N4 and 42% N5, early copepodites were 88% C1 and 11% C2, and late copepodites were 96% C4 and 3% C3, with the remaining 1–2% of copepods excluded from analysis. Nauplii and copepodites were placed individually in 40-mL glass vials and 175-mL polycarbonate bottles, respectively. Incubation bottles were slowly rotated (1 rpm) on a plankton wheel in a constant temperature room at a temperature near ambient with a 12:12 light–dark cycle. After ~ 48 h incubation samples were concentrated on a 35-µm mesh and transferred to 20-mL glass vials, stained with chlorazol black and preserved in 2–5% (final conc.) formaldehyde. The choice of experimental duration was based on earlier measurements of growth rate (Kimmerer et al., 2014b) and preliminary experiments.
Copepods were later identified as alive at preservation (i.e., they had taken up the stain) and identified to stage. About 2% of all 1928 samples were eliminated because the copepod was had failed to take up stain, and 5% because no copepod was present in the sample, probably because it was lost during initial or final transfer. This selection and the elimination of some stages (previous paragraph) left a total of 1772 samples. One experiment with only 6 remaining samples was eliminated, leaving a total of 65 molt-rate measurements with a median of 28 copepods each (range 17–30). To estimate the bias in the recovery process, individual exuviae from recently-molted copepodites and nauplii were incubated and examined as for the molt-rate experiments; 52 of 53 copepodites and 51 of 61 nauplii were recovered.
Calculations
Bayesian analyses were run in WinBugs v. 1.4.3 (Lunn et al., 2000) and other statistical analyses were performed using R version 3.2.3 (R Development Core Team, 2015). Least-squares linear or generalized linear models (McCullagh & Nelder, 1989; Guisan et al., 2002) were used to determine relationships between environmental variables and responses of copepods. After data (e.g., growth rates) and error estimates had been determined, the precision (inverse of variance) was used as a weighting factor in subsequent linear models. Generalized linear models were applied with variance proportional to the mean squared where variance clearly increased with the mean. Error terms, where presented, are either 95% confidence intervals or, for Bayesian analyses, 95% credible intervals.
Stage durations and growth rates in the laboratory and the field were determined using Bayesian hierarchical models (Gelman et al., 2004; Kimmerer & Gould, 2010; Gould & Kimmerer, 2010). The Bayesian approach allowed for use of all the data and the direct determination of uncertainty in parameters and predictions. Uninformative prior distributions were used for all parameters except as noted below. All models were thoroughly tested using data from simulations to ensure recovery of the simulated parameters, and results were checked against values estimated by maximum likelihood. Methods were modified from those in Kimmerer & Gould (2010) and Gould & Kimmerer (2010), and details of the Bayesian approach are given in the online Appendix. Below we describe modifications specific to this study.
Laboratory stage durations
We used a model previously applied to Limnoithona tetraspina (Gould & Kimmerer, 2010), including an asymmetrical logistic function to represent the increasing spread of individual stage durations, and a separation of sexes after C4. Cohorts were treated as blocking factors; the deviations of individual cohorts from the overall mean duration of each stage were small (~ 0.3 d). The model was fitted using the probabilities of a multinomial distribution in which each element is a life stage.
The analysis was done sequentially for Experiments 1 and 2. Prior probability distributions (priors) of stage durations for Experiment 1 were obtained from Kimmerer & Gould (2010) and corrected for temperature using a relationship for egg development (Sullivan & Kimmerer, 2013). Observations showed that the N1 stage lasts ~ 1 ± 1 h (mean ± SD), which was used for the prior distribution for this stage. The mean duration of all nauplius stages from Kimmerer & Gould (2010), less the mean for N1 and the standard deviation of all nauplius stages, was divided by 5 to provide prior probability distributions for each nauplius stage in Experiment 1. Priors for stage durations in Experiment 2 were taken from posterior distributions in Experiment 1. Priors for analysis of durations of Copepodite 5 by sex were taken from posterior distributions for both sexes combined (see online Appendix). Prior distributions for all stage durations were sampled from a normal distribution truncated to include only positive numbers.
Field molt-rates
Field experiments were analyzed using the laboratory-based stage durations and temperature dependence of egg duration (Sullivan & Kimmerer, 2013) to calculate expected molt-rates at field temperatures. In turn, these were used to calculate a development index Φ
where Dlab,T is the laboratory–determined stage duration corrected to field temperature and Dobs is the stage duration calculated from molt frequencies. The calculation followed the method of Gould & Kimmerer (2010), which was modified because we used fixed incubation times in each experiment and because some individuals molted twice (a few molted three times in one experiment). Therefore, the observed number molting in each experiment was fitted using a multinomial rather than a binomial frequency distribution (See online Appendix).
The development index Φ was fitted to chlorophyll concentration in the > 5-µm size fraction using a rectangular hyperbola (Michaelis–Menten or Holling function). This analysis was conducted using a Bayesian approach in which the parameters Φmax (maximum development index) and k (half-saturation constant) were given uninformative prior distributions constrained to be positive and the values of Φ were sampled from their distributions determined in analysis of the molt-rate data. The Bayesian method provided estimates of the parameters as well as prediction errors.
Growth rate
Growth rate was determined from the stage durations and carbon content of each life stage. Carbon content is measured within a stage and is assumed to be the within-stage mean, whereas stage duration is the time between successive molts. The equations for growth rate based on stage durations and mean weights of a series of stages are underdetermined, requiring an additional assumption (Gould & Kimmerer, 2010). For example, Hirst et al. (2005) assumed constant growth between midpoints of stages. Instead, we assumed that growth rate would be constant over a series of life stages; an alternative assumption of growth changing linearly adds uncertainty to the growth–rate estimates because of the additional parameter needed (Gould & Kimmerer, 2010). Growth rate in the field was estimated as the laboratory–determined growth rate of each life stage, reduced by the development index determined in the molt–rate experiments, and adjusted for field temperature. This ignores differences in the temperature responses between growth and development (Forster et al., 2011), which are likely small because of the small range of temperature in our study (see Results).
Results
Long-term monitoring data
The abundance of P. forbesi in fresh water rose rapidly in spring of each year at a rate of ~ 10% d−1 to reach a seasonal maximum in July–September, then began to decline in October (Fig. 2A). The July–September means were narrowly constrained; the third quartile was only 40% more than the first quartile, and the maximum was 2.6 times the minimum (boxplot in Fig. 2A); by contrast, spring abundance varied over several orders of magnitude among years (Fig. 2A).
Patterns of geometric mean abundance of Pseudodiaptomus forbesi copepodites and adults for each year from 1994 to 2016. A Time series of abundance averaged across three freshwater stations (Fig. 1) versus day of year where each line represents data from one year and symbols indicate years of this study. Line types divide data into thirds by mean summer freshwater flow: low flow (95–135 m3 s−1), medium flow (135–235 m3 s−1), and high flow (235–727 m3 s−1). Boxplot insert summarizes mean values for July–September of each year as median, quartiles, and ranges. B and C July–September geometric mean abundance of P. forbesi copepodites and adults for each year from (B) freshwater stations (salinity < 0.5) and (C) stations with salinity between 0.5 and 5 excluding Suisun Marsh and the central to eastern Delta (Fig. 1). Error bars are 95% confidence limits based on all samples from the selected stations, and points for 2011 are shown as open circles. Lines with error bounds are from least-squares models of log of abundance versus flow, weighted by the inverse of variance. Values given in panels B and C are slopes with 95% confidence intervals
The timing of the abundance increase varied with freshwater flow. Specifically, the Julian day when abundance reached 1000 m−3 was related to the log of freshwater flow with a slope of 13.6 ± 5.6 d (95% confidence interval, linear regression, P < 0.0001, R2 = 0.54, 20 df). This may have been due to generally lower minimum abundance at the winter low point, which was negatively related to freshwater flow (slope of log–log relationship = 0.25 ± 0.15, P < 0.001, R2 = 0.41, 19 df because of missing data in winter 1994–1995) but it is difficult to distinguish the effect of low winter abundance from that of a delayed spring increase.
In contrast to winter–spring, the summer abundance maximum in fresh water was invariant with flow (Fig. 2B). In the low-salinity zone, the summer maximum was much lower than in fresh water, but increased with flow in the low-salinity zone, particularly at flows that were exceptionally high for the summer dry season (e.g., 2011 in Fig. 2C).
Laboratory-based development and growth
Total length, carbon, nitrogen, and dry mass of copepodites and adults were closely correlated (Table 2). A straight line fit through the log–log data for carbon versus length gave a slope of 2.43 ± 0.15 and for dry mass a slope of 2.35 ± 0.15 (95% confidence intervals).
Development in P. forbesi was not isochronal as shown by the mean durations and credible intervals of each stage from the Bayesian analysis (Fig. 3). N1 developed quickly, N2 was extended, and N6 took slightly longer to develop than N3–N5. Durations of late copepodite stages were the longest, especially for females (Fig. 3). It took adult females 1.8 d to become ovigerous after the final molt. The total duration of all nauplius stages combined (Dn) was 3.13 ± 0.11 d, while that of copepodites (Dc) was 4.65 ± 0.05 d for females and 4.55 ± 0.05 d for males. Total stage durations were 7.8 ± 0.13 and 7.7 ± 0.13 d respectively, and the ratio Dc/Dn was 1.49 ± 0.06. Laboratory growth rates were high and similar between nauplii and early copepodites, and somewhat lower in C4–C5 females (Table 2). C4–C5 males had lower growth rates than the females despite the males’ shorter stage durations.
Laboratory stage duration (d) by stage for Pseudodiaptomus forbesi at 22°C. Values are means ± 95% credible intervals, also shown by error bars. F and M, female and male. OV, time to develop the first clutch of eggs after females molt to adult stage. Data based on a median of 54 (nauplii) or 68 (copepodites) copepods per ~ -12-h time step taken from each of the triplicate containers
Field sampling
Freshwater flow during summer–autumn was low, as is typical of the dry season (Fig. 4A). The year 2011 ranked only 42 of the 60 water years from 1956 through 2015 in flow during the wet season (November–April), while its mean summer flow was relatively high during our study, and about twice the flows observed in 2010 and 2012. The higher flow in 2011 resulted in a seaward displacement of the salinity field, such that salinity at station 1 (most seaward) was substantially lower in 2011 than in either 2010 or 2012, when salinity values were similar (Table 3). Water temperature was ~ 22°C during most of the summer in 2011 and 2012 and slightly lower during the earlier part of the sampling period during 2010 (Fig. 4B). Freshwater flow was uncorrelated with temperature but there was a negative correlation between the log of flow and solar radiation (r = −0.47, 95% confidence interval −0.74, −0.06).
Freshwater flow and temperature during field sampling. A Freshwater flow as calculated net outflow from the Delta (http://www.water.ca.gov/dayflow/). Lines indicate values from each of the study years. Symbols at top indicate timing of sampling events each year on each river; filled lower half, transect; filled upper half, molt-rate experiment. B Temperature during the same period from a station at Antioch (Fig. 1), obtained from the California Data Exchange Center (http://cdec.water.ca.gov/)
Patterns of abundance of P. forbesi from the 2010–2012 transects differed sharply between rivers and somewhat less among years (Fig. 5A, B). Abundance in the Sacramento River was highest at stations 3–7, declined at the more seaward (lower-numbered) stations, and dropped sharply at station 8 (Fig. 5A). This is the first station in the Sacramento River upstream of its confluence with Cache Slough (Fig. 1). Stations above that point on the Sacramento River were in a more riverine and less estuarine region, and were more influenced by residual and less by tidal flows than the stations below the confluence. At stations 9–12 P. forbesi was barely detectable.
Conditions along transects in 2010–2012 in the Sacramento (A, C, E) and San Joaquin Rivers (B, D, F), where station 1 is most seaward (Fig. 1). A, B, Abundance per unit volume of all stages of Pseudodiaptomus forbesi with areas of symbols proportional to the total number of copepods counted, range 1–13020; C, D, Proportion of gross life stages averaged over transect data from dry years 2010 and 2012; E, F Chlorophyll concentration in particles larger than 5 µm. Line types and symbols in A, B, E, F indicate years and transects 1 and 2
In the San Joaquin River abundance was generally highest at the most landward stations, and exceeded the maxima in the Sacramento River during every pair of transects (Fig. 5B). Abundance declined from landward to seaward, and in 2010 and 2012 total abundance was about 100-fold lower at the most seaward station than at the freshwater peak in abundance. In 2011 the decline in abundance with distance along the transects was much gentler, resulting in about a 10-fold decrease along each transect. This was because the salinity field had shifted seaward in 2011 relative to the other years (Table 3), and the zooplankton shifted with it.
In both rivers, the proportions of life stages varied along the transects, although that variation was small in 2011 (not shown) because the declining limb of abundance to seaward was not captured. In the upper part of the Sacramento River transects in 2010 and 2012 almost all the copepods were nauplii (Fig. 5C), but counts were so low that these values are unreliable. Otherwise, in both rivers in 2010 and 2012 the relative abundance of nauplii, and to some extent copepodites, declined and the relative abundance of adults increased, going from fresh water into the low-salinity zone.
Chlorophyll concentrations in the > 5 µm size fraction were mostly below 5 µg Chl L−1 except for one high value on the first transect in 2012 in the Sacramento River (Fig. 5E), the seaward-most sample in the second transect in 2011 and some very high values on the second transect in 2012 in the San Joaquin River (Fig. 5F). Otherwise, the chlorophyll values were generally higher on the first transect of 2011 than any others. The means along all other transects were not over 3 µg L−1, and those from 2010 did not exceed 1 µg Chl L−1.
Reproduction and growth in the field
Egg production rate (EPR) differed among years, with the highest mean value in 2012 and similar values in 2010 and 2011 (Fig. 6). Means varied among individual transects within years, so between-year differences were not tested. Otherwise, EPR was invariant with both chlorophyll (Fig. 6A) and freshwater flow (Fig. 6B).
Egg production rate of Pseudodiaptomus forbesi during 2010–2012, plotted against A Chlorophyll concentration in particles larger than 5 µm and B Freshwater flow. Symbols are means for each transect including only stations in which > 5 females were counted. Error bars are 95% confidence intervals of the mean. Symbols indicate rivers (legend) and year: 2010 (black), 2011 (red), 2012 (green)
Stage durations of P. forbesi in the field were about twice as long as temperature-corrected laboratory stage durations for copepodites (development index Φ ~ 0.5) and about four times as long for nauplii (Φ ~ 0.25, Fig. 7). The Φ value of late copepodites was related to chlorophyll concentration by a rectangular hyperbola (Fig. 7A) with an asymptotic Φ value of 0.76 ± 0.06. Fits for the other two stages were poor and estimates of the half-saturation constant were highly uncertain. Asymptotic Φ values were lowest for nauplii (0.27 ± 0.05) and next lowest for early copepodites (0.43 ± 0.02) with no overlap of credible intervals among the stages. Residuals from the chlorophyll analyses were similar between the two rivers and were about 0.10 ± 0.06 higher in the low-salinity zone than in fresh water. Development indices of all stages were unrelated to freshwater flow (Fig. 7 B, D, F).
Development index Φ (Eq. 1 ) for Pseudodiaptomus forbesi during 2010–2012, plotted against A, C, E, chlorophyll larger than 5 µm; and B, D, F, freshwater flow. A, B, late copepodites; C, D, early copepodites; E, F, nauplii. A development index of 1 indicates growth at the rate determined in the laboratory with abundant food, corrected for field temperature. Symbols indicate the river and salinity region, and error bars are 95% confidence intervals of the mean. Line and shading in A give predictions of Φ with 95% confidence bounds determined from chlorophyll concentration; parameters in text
Growth rates ranged from 0.03 to 0.27 d−1 (Fig. 8), and were lower for nauplii than for the other two stages (difference 0.07 ± 0.03, 95% confidence interval, generalized linear model with identity link function and variance proportional to mean squared). Variability within years and rivers was high and differences among years were negligible; adding year or station to the above model did not improve the fit.
Growth rates of nauplii and copepodites under field conditions, calculated from molt-rate results and corrected for field temperature. Values are shown by year and adjusted slightly by day of the year for separation. Symbols indicate river and salinity region as in Fig. 7
Discussion
Neither egg production rate nor stage durations nor, by implication, growth rate of Pseudodiaptomus forbesi varied with freshwater flow (Figs. 6B, 7B, D, F), and egg production rate was unrelated to chlorophyll concentration (Fig. 6A). Stage durations, expressed as the development index Φ, were weakly related to chlorophyll concentration in the > 5 µm fraction for late copepodites only (Fig. 7A). Egg production and development rates were generally low, as previously found in the low-salinity zone (Kimmerer et al., 2014b), yet this copepod is the most abundant mesozooplankton species in fresh water and a key food for fishes in both fresh and low-salinity water. Below, we discuss the patterns of development and growth in the laboratory and the field, and then the influences of environment including freshwater flow on development, reproduction, and spatial and temporal patterns of abundance.
Laboratory-determined development and growth
Pseudodiaptomus forbesi follows a pattern of development typical of most calanoid copepods (Landry, 1983; Hart, 1990): the N1 stage is very brief, N2 is prolonged and presumably is the first-feeding stage, late copepodites develop more slowly than earlier stages, and males develop faster than females but to a smaller final size. Similar patterns have been seen in other Pseudodiaptomus species (Uye et al., 1983). The ratio of duration of copepodite to nauplius stages (Dc/Dn) for P. forbesi (1.49 ± 0.06) fell between values reported for P. hessei (1.15–1.46 at four temperatures; Jerling & Wooldridge, 1991) and P. marinus (1.7, Uye et al., 1983). Other estuarine genera such as Eurytemora have similar ratios (Ban, 1994; Devreker et al., 2007).
Isochronal development, most prominently seen in Acartia species, has been explained as an adaptation to delay development in the face of size-selective visual predation on larger life stages in estuaries (Miller et al., 1977; Landry, 1983; Hart, 1990). Estuarine populations of Pseudodiaptomus and Eurytemora would presumably be exposed to a similar predatory risk. However, larger individuals of these genera are often most abundant in the estuarine turbidity maximum (Cordell et al., 1992; Islam et al., 2005; Lloyd et al., 2013) or have a demersal habit by which they remain near or on the bottom by day, presumably to avoid visual predation (Fancett & Kimmerer, 1985; Vuorinen, 1987), a behavior that may be enhanced in clear water (Kimmerer & Slaughter, 2016). Thus demersal behavior may be an alternative life-history strategy to isochronal development in the face of strongly size-selective predation (Miller et al., 1977).
The under-determined set of equations describing growth (see online Appendix) required additional assumptions about patterns of growth within stages. This problem is equivalent to that for mortality, which is often calculated using the Ratio method by which mortality is determined between successive pairs of stages (Gentleman et al., 2012). Alternatively, mortality can be assumed constant across several stages (Kimmerer, 2015), as we have done here for growth. The disadvantage of constant growth rate is the lack of information it provides about growth rates among stages. The practical advantage is that it avoids the poor precision inherent in analyses including only a pair of stages.
Laboratory growth rates of Pseudodiaptomids have been determined in only a few studies. Specific growth rates of P. marinus in the Sea of Japan were 0.23 ± 0.08 d−1 for nauplii and 0.36 ± 0.15 d−1 for copepodites at 20°C (Uye et al., 1983). These values are equivalent to 0.29 and 0.46 d−1, respectively, when adjusted using the temperature relationship of Sullivan & Kimmerer, (2013). This estimate of specific growth rate in P. marinus nauplii is ~ half of our value for P. forbesi, while copepodite growth rates are similar between studies (Table 2). Specific growth rates of P. annandalei nauplii at 20°C ranged from 0.16 to 0.60 d−1 while those of copepodites decreased from 0.8 d−1 in C1 to 0.26 d−1 in C5 (Li et al., 2009, as P. dubia). However, stage duration was determined from very low counts during incubation and the analysis assumed linear growth within each stage (Li et al., 2009), making comparison with our results difficult. Thus, general patterns of growth in Pseudodiaptomus species remain to be determined.
The Bayesian approach for growth rate estimates has three advantages over traditional approaches. First, it is easy to incorporate uncertainty arising from estimation error in both body mass and stage durations. Propagation of these errors can introduce substantial uncertainty in growth rate estimates that should not be ignored. Second, it simplifies the calculations, which would otherwise require optimization or a resampling procedure. Third, it provides growth rate estimates with full statistical distributions, which are available for use in subsequent calculations.
Field development, growth, and food limitation
Low food supply may affect copepod population dynamics by limiting the nutrition available for growth and development or gamete production in adults. The energy available for growth and reproduction may also be reduced by heightened metabolic demands for osmoregulation or in response to stress (Lee et al., 2013; Hammock et al., 2015). Thus, food limitation can be aggravated by stressful environmental conditions.
Although estuarine and coastal waters are typically considered more productive than oceanic waters, recent studies have shown wide variability in the levels and seasonality of primary production and phytoplankton biomass, implying similar variability in patterns of food availability to copepods. For example, a review of primary production estimates from well-studied estuarine waters showed that about 30% of the records could be classified as oligotrophic (Cloern et al., 2014). The interannual median of annual mean chlorophyll concentrations was above 10 µg L−1 in only 42 of 154 estuarine and coastal ecosystems, and interannual variability in the longer records was ~ 10–fold (Cloern & Jassby, 2008).
The literature on food limitation of estuarine and coastal copepods seems too sparse to support strong predictions about whether a particular population might be food-limited. Most of the available information is on Acartia, a broadcast-spawning genus ubiquitous in temperate estuaries of the northern hemisphere. For species in this genus, half-saturation constants Km of the Michaelis–Menten or Holling, (1966) equation for reproductive or growth rates have ranged from ~ 1 to 6 µg Chl L−1 (Bunker & Hirst, 2004; Kimmerer et al., 2005). Arbitrarily defining food limitation as any rate below 80% of the maximum, food limitation would occur at four times the Km value or 4–24 µg Chl L−1. Coupled with the estimates of chlorophyll concentrations in estuaries discussed above, this implies that food limitation of Acartia species should be commonplace in unproductive estuaries.
Far less information is available on food limitation in sac-spawning copepods. Reviews of reproduction and growth in copepods showed that sac-spawners reproduce more slowly and appear less likely to be food-limited than broadcast spawners (Hirst & Bunker, 2003; Bunker & Hirst, 2004). However, that conclusion is based upon only two genera, Oithona and Pseudocalanus, the latter from a single study.
Few studies of estuarine sac-spawners provide parameters describing feeding, reproduction, or growth under a wide range of conditions. Some results have been consistent with the general pattern discussed above: feeding rate (Irigoien et al., 1996 for the Gironde estuary) and egg production rate (Lloyd et al., 2013 for Chesapeake Bay) of the sac-spawning species complex Eurytemora affinis was less often food-limited than corresponding rates of co-occurring Acartia species. Other reports on members of the E. affinis complex have been less consistent with the assumed patterns of low growth and reproductive rate, with laboratory egg production rates of 34 ♀−1 d−1 at 15 and 20°C in E. affinis from Lake Biwa, Japan (Ban, 1994), and 38 ♀−1 d−1 in E. affinis from Chesapeake Bay (Devreker et al., 2007), values that approach food-saturated rates for broadcast spawners (Bunker & Hirst, 2004). Several reports have documented food limitation of E. affinis in unproductive estuaries, e.g., in egg production in the Gironde (Burdloff et al., 2000) and somatic growth in the Westernscheldt estuary (Escaravage & Soetaert, 1995).
A small but growing literature on the speciose estuarine genus Pseudodiaptomus shows generally low maximum egg production rates in laboratory experiments: e.g., 13 nauplii ♀−1 d−1 in cultured P. pelagicus Herrick, 1884 (Ohs et al., 2010), 12 eggs ♀−1 d−1 in P. annandalei (Beyrend-Dur et al., 2011), and 9–10 eggs ♀−1 d−1 in P. australiensis Walter, 1987 (Gusmao & McKinnon, 2016). Field measurements showed a mean egg production rate of ~ 7 eggs ♀−1 d−1 for P. marinus in the Inland Sea of Japan during summer, where food saturation was inferred from a lack of correlation with chlorophyll (Liang & Uye, 1997). In contrast, P. hessei produced up to 37 eggs ♀−1 d−1 in the Kariega estuary, South Africa, and egg production was positively related to chlorophyll and to fatty acid content of the food (Noyon & Froneman, 2013). Our results for P. forbesi (Fig. 6) show low egg production rates with a mean of 1.5 eggs ♀−1 d−1 and no response to chlorophyll. We have not determined the maximum egg production rate, although the observed rate is probably well below the maximum as inferred from late copepodite growth rate for this species in the low-salinity zone in 2006–2007 (Kimmerer et al., 2014b).
Food limitation of P. forbesi in this study is demonstrated by the persistently low development index and the relationship of the index for late copepodites to chlorophyll concentration (Fig. 7A). The relationships for all stages were very scattered and the data were mostly confined to the low-chlorophyll end of the range. However, the poor fit of the relationships and the fact that few of the indices approached 1 suggest that chlorophyll concentration, even in the > 5-µm size fraction, is a poor proxy for food supply for this species. This is no surprise given the breadth of diets typically found in calanoid copepods (Kleppel, 1993). The development index of nauplii was the most severely limited and this life stage showed no real response to chlorophyll concentration. This is unusual in that copepod nauplii are usually found to be less food-limited than copepodites (Hart, 1990; Hopcroft & Roff, 1998; Finlay & Roff, 2006).
An alternative explanation for the persistently low maximum development indices is that the copepods were chronically stressed and unable to grow at their maximum rate. We cannot rule out effects of stress, although salinity stress is unlikely given the rather small differences in development indices between fresh and brackish water. Toxic stress is always a possibility in this estuary with its urban and agricultural land uses, and stress due to ingestion of Microcystis aeruginosa is likely (Lehman et al., 2013).
Persistently low abundance of food, principally phytoplankton, in the San Francisco Estuary previously has been attributed to high turbidity, grazing by introduced clams (Kimmerer & Thompson, 2014), and possibly nutrient interactions (Parker et al., 2012). The co-occurring cyclopoid Limnoithona tetraspina may be food-limited as well (Bouley & Kimmerer, 2006; Gould & Kimmerer, 2010), and chronic food limitation of zooplankton seems to be the rule for zooplankton throughout the estuary (Orsi & Mecum, 1996; Müller-Solger et al., 2002; Kimmerer et al., 2005).
Abundance and flow responses
Previous reports documented positive relationships between freshwater flow into the SFE and abundance of several fish and macroinvertebrate species (Jassby et al., 1995; Kimmerer et al., 2013). Such relationships appear to be common in estuaries around the world and may arise through a wide variety of mechanisms (Drinkwater & Frank, 1994; Kimmerer, 2002; Alber, 2002). A commonly cited group of mechanisms for positive effects of flow on abundance of estuarine biota is through stimulation of organic input to the estuary by elevated flow, either through advective transport of organic matter or nutrients (Nixon et al., 1986), or through enhanced stratification due to elevated runoff (Cloern, 1984). In the northern, river-dominated SFE, responses of abundance to flow are much stronger for fish than for zooplankton (Kimmerer, 2002). This may suggest that mechanisms for positive relationships of fish abundance to flow do not arise through trophic links. This is further supported by our finding that growth and reproduction of P. forbesi appear to be unresponsive to flow.
Summer abundance in the freshwater population center of P. forbesi was invariant with flow (Fig. 2B) and the summer mean abundance was constrained to a strikingly narrow range (boxplot in Fig. 2A). These years include a range of freshwater flows (Fig. 2B), although summer flows are greatly reduced from winter to spring. These years also include times of strong blooms of the toxic cyanobacterium Microcystis aeruginosa and times without blooms (Lehman et al., 2013), times of high rates of water diversion relative to inflow, and times of high and low abundance of planktivorous fishes (Sommer et al., 2007). We are mystified by this narrow range of abundance and what strong feedback mechanisms must exist to maintain it.
Freshwater flow may have a role in subsidizing the zooplankton of the low-salinity zone, probably through advection. In contrast with abundance in fresh water, summer abundance of P. forbesi in the low-salinity zone increased with increasing flow (Fig. 2C). This is not due to a stimulation of growth or reproduction as has been reported for other estuaries; neither responded to flow (Figs. 6, 7) and chlorophyll concentration was unrelated to flow and was highest during the drought year of 2012 (Fig. 5E, F). Rather, this pattern suggests either a reduction in mortality in the low-salinity zone, or an increasing role of advection in transport of copepods from their freshwater population center to the low-salinity zone. These two possibilities will be examined in a subsequent report.
The rapid increase in abundance of P. forbesi during spring is likely a result of increasing temperature, as this species originated from tropical to subtropical regions (Orsi & Walter, 1991) and is slow to reproduce below a temperature of ~ 16°C (L. Sullivan, SFSU, pers. comm.; see Sullivan & Kimmerer, 2013). This species undergoes a similar increase in the Columbia River with peak abundance in August when temperature reaches ~ 20°C (Dexter et al. 2015). Temperature in the upper SFE exceeds 20°C during June–September and P. forbesi is abundant through this period (Fig. 2). Thus the difference in phenology of this species between the two estuaries is likely related to the duration of temperature suitable for this species.
The timing of the spring increase varied with freshwater flow, with higher flow delaying the increase (Fig. 2A). This delay was unrelated to temperature or solar radiation (a proxy for cloud cover which would reduce primary production during stormy periods) and followed generally lower abundance in winter during wet years. The lower winter abundance and delayed spring increase are most likely due to higher advective losses during periods of high flow combined with sluggish development of the copepods at low winter temperatures. Advective losses due to high flow can overcome the ability of tidal vertical migration to hold copepods in place except in deep areas with enough of a salinity gradient to cause strong stratification (Simons et al., 2006; Kimmerer et al., 2014a).
Spatial subsidy and the role of predation
The spatial pattern of abundance of P. forbesi in the upper SFE, coupled with strong tidal dispersion and advection that increases with freshwater flow, result in a subsidy of copepods to the low-salinity zone. Spatial subsidies are an important feature of ecosystems and can take many forms including nutrients, organic matter, and organisms (Polis et al., 1997). Spatial subsidies in estuarine and coastal plankton assemblages appear to be common, e.g., in supplying phytoplankton from estuaries to grazers on the open coast (Savage et al., 2012) or zooplankton from open waters to filter-feeders on coral reefs (Genin, 2004). The magnitude of a spatial subsidy in plankton is a function of the advective and dispersive transport and the residence time and population growth rate of the source population (Cloern, 2007).
The subsidy of copepods from fresh water to the low-salinity zone is similar to that identified for phytoplankton biomass, which is usually higher in fresh water than in low-salinity water (Kimmerer & Thompson, 2014). This pattern was attributed to grazing by the clam Potamocorbula amurensis, in addition to grazing by micro- and mesozooplankton, in low-salinity waters (Kimmerer & Thompson, 2014).
Why is P. forbesi so much more abundant in fresh water than in the low-salinity zone? This distribution pattern is not due to salinity tolerance, as the optimum salinity for reproduction of this species is ~ 5, and nauplii can survive well at salinity ~ 12 (Kayfetz & Kimmerer, 2017). Egg production was no higher (Fig. 6), and growth rate slightly lower (discussed above), in fresh water than brackish. Clams in brackish water consume copepod nauplii but their consumption rate is lower than on phytoplankton because of the copepods’ escape responses (Kimmerer & Lougee, 2015); however, the copepods have longer population turnover times than phytoplankton and are also vulnerable to predation by the introduced predatory copepod Acartiella sinensis (Slaughter et al., 2016). The combined mortality to P. forbesi nauplii was estimated at about 26% d−1 (Kimmerer & Lougee, 2015; Slaughter et al., 2016; Kayfetz & Kimmerer, 2017). This mortality rate would be unsustainable in a closed population with reproductive rates < 2% d−1 (Fig. 6, assuming a 50:50 sex ratio of offspring). Thus, it appears that the seaward limit of the distribution of P. forbesi is set by the interplay between the magnitude of the spatial subsidy and losses to predation.
If this assessment is correct, it provides yet another example of the dominance of predation in structuring marine and estuarine assemblages (e.g., Heck & Valentine, 2007). The influence of predation on size and species composition of zooplankton has been known for a long time (Brooks & Dodson, 1965; Hall et al., 1976), mainly from studies in lakes. Similar inferences can be made in estuaries only indirectly and with consideration of other influences such as salinity, flow, and relative movements and abundance patterns of predators and prey.
References
Alber, M., 2002. A conceptual model of estuarine freshwater inflow management. Estuaries 25: 1246–1261.
Ban, S., 1994. Effect of temperature and food concentration on post-embryonic development, egg production and adult body size of calanoid copepod Eurytemora affinis. Journal of Plankton Research 16: 721–735.
Bennett, W. A., 2005. Critical assessment of the delta smelt population in the San Francisco Estuary, California. San Francisco Estuary and Watershed Science 3: Article 1.
Beyrend-Dur, D., R. Kumar, T. R. Rao, S. Souissi, S. H. Cheng & J. S. Hwang, 2011. Demographic parameters of adults of Pseudodiaptomus annandalei (Copepoda: Calanoida): Temperature-salinity and generation effects. Journal of Experimental Marine Biology and Ecology 404: 1–14.
Bouley, P. & W. J. Kimmerer, 2006. Ecology of a highly abundant, introduced cyclopoid copepod in a temperate estuary. Marine Ecology Progress Series 324: 219–228.
Bowen, A., G. Rollwagen-Bollens, S. M. Bollens & J. Zimmerman, 2015. Feeding of the invasive copepod Pseudodiaptomus forbesi on natural microplankton assemblages within the lower Columbia River. Journal of Plankton Research 37: 1089–1094.
Brooks, J. L. & S. I. Dodson, 1965. Predation, body size, and composition of plankton. Science 150: 28–35.
Bryant, M. E. & J. D. Arnold, 2007. Diets of age-0 striped bass in the San Francisco Estuary, 1973–2002. California Fish and Game 93: 1–22.
Bunker, A. J. & A. G. Hirst, 2004. Fecundity of marine planktonic copepods: global rates and patterns in relation to chlorophyll a, temperature and body weight. Marine Ecology Progress Series 279: 161–181.
Burdloff, D., S. Gasparini, B. Sautour, H. Etcheber & J. Castel, 2000. Is the copepod egg production in a highly turbid estuary (the Gironde, France) a function of the biochemical composition of seston? Aquatic Ecology 34: 165–175.
Cloern, J. E., 1984. Temporal dynamics and ecological significance of salinity stratification in an estuary (South San Francisco Bay, USA). Oceanologica Acta 7: 137–141.
Cloern, J. E., 2007. Habitat connectivity and ecosystem productivity: implications from a simple model. American Naturalist 169: E21–E33.
Cloern, J. E. & A. D. Jassby, 2008. Complex seasonal patterns of primary producers at the land-sea interface. Ecology Letters 11: 1294–1303.
Cloern, J. E. & A. D. Jassby, 2012. Drivers of change in estuarine-coastal ecosystems: discoveries from four decades of study in San Francisco Bay. Reviews in Geophysics 50: 1–33.
Cordell, J. R., C. A. Morgan & C. A. Simenstad, 1992. Occurrence of the Asian calanoid copepod Pseudodiaptomus inopinus in the zooplankton of the Columbia River Estuary. Journal of Crustacean Biology 12: 260–269.
Cloern, J. E., S. Q. Foster & A. E. Kleckner, 2014. Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences 11: 2477–2501.
Devreker, D., S. Souissi, J. Forget-Leray & F. Leboulenger, 2007. Effects of salinity and temperature on the post-embryonic development of Eurytemora affinis (Copepoda; Calanoida) from the Seine estuary: a laboratory study. Journal of Plankton Research 29: 117–133.
Dexter, E., S. M. Bollens, G. Rollwagen-Bollens, J. Emerson & J. Zimmerman, 2015. Persistent vs. ephemeral invasions: 8.5 years of zooplankton community dynamics in the Columbia River. Limnology and Oceanography 60: 527–539.
Drinkwater, K. F. & K. T. Frank, 1994. Effects of river regulation and diversion on marine fish and invertebrates. Aquatic Conservation: Marine and Freshwater Ecosystems 4: 135–151.
Edmondson, W. T., G. C. Anderson & G. W. Comita, 1962. Reproductive rate of copepods in nature and its relation to phytoplankton population. Ecology 43: 625–634.
Escaravage, V. & K. Soetaert, 1995. Secondary production of the brackish copepod communities and their contribution to the carbon fluxes in the Western scheldt Estuary (the Netherlands). Hydrobiologia 311: 103–114.
Fancett, M. S. & W. J. Kimmerer, 1985. Vertical migration of the demersal copepod Pseudodiaptomus as a means of predator avoidance. Journal of Experimental Marine Biology and Ecology 88: 31–43.
Finlay, K. & J. C. Roff, 2006. Ontogenetic growth rate responses of temperate marine copepods to chlorophyll concentration and light. Marine Ecology Progress Series 313: 145–156.
Forster, J., A. G. Hirst & G. Woodward, 2011. Growth and development rates have different thermal responses. American Naturalist 178: 668–678.
Gelman, A., J. B. Carlin, H. S. Stern & D. B. Rubin, 2004. Bayesian Data Analysis, 2nd ed. CRC Press, Boca Raton, FL.
Genin, A., 2004. Bio-physical coupling in the formation of zooplankton and fish aggregations over abrupt topographies. Journal of Marine Systems 50: 3–20.
Gentleman, W. C., P. Pepin & S. Doucette, 2012. Estimating mortality: clarifying assumptions and sources of uncertainty in vertical methods. Journal of Marine Systems 105: 1–19.
Gould, A. L. & W. J. Kimmerer, 2010. Development, growth, and reproduction of the cyclopoid copepod Limnoithona tetraspina in the upper San Francisco Estuary. Marine Ecology Progress Series 412: 163–177.
Guisan, A., T. C. Edwards & T. Hastie, 2002. Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecological Modelling 157: 89–100.
Gusmao, F. & A. D. McKinnon, 2016. Egg production and naupliar growth of the tropical copepod Pseudodiaptomus australiensis in culture. Aquaculture Research 47: 1675–1681.
Hall, D. J., S. T. Threlkeld, C. W. Burns & P. H. Crowley, 1976. The size efficiency hypothesis and the size structure of zooplankton communities. Annual Review of Ecology and Systematics 7: 177–208.
Hammock, B. G., J. A. Hobbs, S. B. Slater, S. Acuna & S. J. Teh, 2015. Contaminant and food limitation stress in an endangered estuarine fish. Science of the Total Environment 532: 316–326.
Hart, R. C., 1990. Copepod post-embryonic durations - pattern, conformity, and predictability - The realities of isochronal and equiproportional development, and trends in the copepodid- naupliar duration ratio. Hydrobiologia 206: 175–206.
Heck, K. L. & J. F. Valentine, 2007. The primacy of top-down effects in shallow benthic ecosystems. Estuaries and Coasts 30: 371–381.
Hirst, A. G. & A. J. Bunker, 2003. Growth of marine planktonic copepods: global rates and patterns in relation to chlorophyll a, temperature, and body weight. Limnology and Oceanography 48: 1988–2010.
Hirst, A. G., W. T. Peterson & P. Rothery, 2005. Errors in juvenile copepod growth rate estimates are widespread: problems with the Moult Rate method. Marine Ecology Progress Series 296: 263–279.
Hirst, A. G., J. E. Keister, A. J. Richardson, P. Ward, R. S. Shreeve & R. Escribano, 2014. Re-assessing copepod growth using the Moult Rate method. Journal of Plankton Research 36: 1224–1232.
Holling, C. S., 1966. The functional response of invertebrate predators to prey density. Memoirs of the Entomological Society of Canada 98: 5–86.
Hopcroft, R. R. & J. C. Roff, 1998. Zooplankton growth rates: the influence of size in nauplii of tropical marine copepods. Marine Biology 132: 87–96.
Irigoien, X., J. Castel & S. Gasparini, 1996. Gut clearance rate as predictor of food limitation situations. Application to two estuarine copepods: Acartia bifilosa and Eurytemora affinis. Marine Ecology Progress Series 131: 159–163.
Islam, M. S., H. Ueda & M. Tanaka, 2005. Spatial distribution and trophic ecology of dominant copepods associated with turbidity maximum along the salinity gradient in a highly embayed estuarine system in Ariake Sea, Japan. Journal of Experimental Marine Biology and Ecology 316: 101–115.
Jassby, A. D., W. J. Kimmerer, S. G. Monismith, C. Armor, J. E. Cloern, T. M. Powell, J. R. Schubel & T. J. Vendlinski, 1995. Isohaline position as a habitat indicator for estuarine populations. Ecological Applications 5: 272–289.
Jerling, H. L. & T. H. Wooldridge, 1991. Population dynamics and estimates of production for the calanoid copepod Pseudodiaptomus hessei in a warm temperate estuary. Estuarine, Coastal, and Shelf Science 33: 121–135.
Kayfetz, K. & W. Kimmerer, 2017. Abiotic and biotic controls on the copepod Pseudodiaptomus forbesi in the upper San Francisco Estuary. Marine Ecology Progress Series 581: 85–101.
Kimmerer, W. J., 2002. Physical, biological, and management responses to variable freshwater flow into the San Francisco Estuary. Estuaries 25: 1275–1290.
Kimmerer, W. J., 2015. Mortality estimates of stage-structured populations must include uncertainty in stage duration and relative abundance. Journal of Plankton Research 37: 939–952.
Kimmerer, W. & A. Gould, 2010. A Bayesian approach to estimating copepod development times from stage frequency data. Limnology and Oceanography: Methods 8: 118–126.
Kimmerer, W. J. & L. A. Lougee, 2015. Bivalve grazing causes substantial mortality to an estuarine copepod population. Journal of Experimental Marine Biology and Ecology 473: 53–63.
Kimmerer, W. J. & A. D. McKinnon, 1987. Growth, mortality, and secondary production of the copepod Acartia tranteri in Westernport Bay, Australia. Limnology and Oceanography 32: 14–28.
Kimmerer, W. & A. Slaughter, 2016. Fine-scale distributions of zooplankton in the Northern San Francisco Estuary. San Francisco Estuary and Watershed Science 14: Article 1.
Kimmerer, W. J. & J. K. Thompson, 2014. Phytoplankton growth balanced by clam and zooplankton grazing and net transport into the low-salinity zone of the San Francisco Estuary. Estuaries and Coasts 37: 1202–1218.
Kimmerer, W. J., M. H. Nicolini, N. Ferm & C. Peñalva, 2005. Chronic food limitation of egg production in populations of copepods of the genus Acartia in the San Francisco Estuary. Estuaries 28: 541–550.
Kimmerer, W. J., M. L. MacWilliams & E. S. Gross, 2013. Variation of fish habitat and extent of the low-salinity zone with freshwater flow in the San Francisco Estuary. San Francisco Estuary and Watershed Science 11: Article 3.
Kimmerer, W. J., E. S. Gross & M. L. MacWilliams, 2014a. Tidal migration and retention of estuarine zooplankton investigated using a particle-tracking model. Limnology and Oceanography 59: 901–906.
Kimmerer, W. J., T. R. Ignoffo, A. M. Slaughter & A. L. Gould, 2014b. Food-limited reproduction and growth of three copepod species in the low-salinity zone of the San Francisco Estuary. Journal of Plankton Research 36: 722–735.
Kleppel, G. S., 1993. On the diets of calanoid copepods. Marine Ecology Progress Series 99: 183–195.
Landry, M. R., 1983. The development of marine calanoid copepods with comment on the isochronal rule. Limnology and Oceanography 28: 614–624.
Laprise, R. & J. J. Dodson, 1993. Nature of environmental variability experienced by benthic and pelagic animals in the St. Lawrence Estuary, Canada. Marine Ecology Progress Series 94: 129–139.
Lee, C. E., W. E. Moss, N. Olson, K. F. Chau, Y. M. Chang & K. E. Johnson, 2013. Feasting in fresh water: impacts of food concentration on freshwater tolerance and the evolution of food x salinity response during the expansion from saline into fresh water habitats. Evolutionary Applications 6: 673–689.
Lehman, P. W., K. Marr, G. L. Boyer, S. Acuna & S. J. Teh, 2013. Long-term trends and causal factors associated with Microcystis abundance and toxicity in San Francisco Estuary and implications for climate change impacts. Hydrobiologia 718: 141–158.
Li, C. L., X. X. Luo, X. H. Huang & B. H. Gu, 2009. Influences of temperature on development and survival, reproduction and growth of a calanoid copepod (Pseudodiaptomus dubia). The Scientific World Journal 9: 866–879.
Liang, D. & S. Uye, 1997. Seasonal reproductive biology of the egg-carrying calanoid copepod Pseudodiaptomus marinus in a eutrophic inlet of the Inland Sea of Japan. Marine Biology 128: 409–414.
Lloyd, S. S., D. T. Elliott & M. R. Roman, 2013. Egg production by the copepod, Eurytemora affinis, in Chesapeake Bay turbidity maximum regions. Journal of Plankton Research 35: 299–308.
Lunn, D. J., A. Thomas, N. Best & D. Spiegelhalter, 2000. WinBUGS - A Bayesian modelling framework: Concepts, structure, and extensibility. Statistics and Computing 10: 325–337.
McCullagh, P. & J. Nelder, 1989. Generalized Linear Models. Chapman and Hall, London.
Meng, L. & J. J. Orsi, 1991. Selective predation by larval striped bass on native and introduced copepods. Transactions of the American Fisheries Society 120: 187–192.
Miller, C. B., J. K. Johnson & D. R. Heinle, 1977. Growth rules in the marine copepod genus Acartia. Limnology and Oceanography 22: 326–335.
Moyle, P. B., L. R. Brown, J. R. Durand & J. A. Hobbs, 2016. Delta smelt: life history and decline of a once-abundant species in the San Francisco Estuary. San Francisco Estuary and Watershed Science 14: Article 2.
Moyle, P. B. & R. A. Leidy, 1992. Loss of biodiversity in aquatic ecosystems: evidence from fish faunas. In Fiedler, P. L. & S. K. Jain (eds), Conservation Biology: The Theory and Practice of Nature Conservation, Preservation, and Management. Chapman & Hall, New York, NY: 127–170.
Müller-Solger, A. B., A. D. Jassby & D. Müller-Navarra, 2002. Nutritional quality of food resources for zooplankton (Daphnia) in a tidal freshwater system (Sacramento-San Joaquin River Delta). Limnology and Oceanography 47: 1468–1476.
Nixon, S. W., C. A. Oviatt, J. Frithsen & B. Sullivan, 1986. Nutrients and the productivity of estuarine and coastal marine systems. Journal of the Limnological Society of South Africa 12: 43–71.
Nobriga, M. L., 2002. Larval delta smelt diet composition and feeding incidence: environmental and ontogenetic influences. California Fish and Game 88: 149–164.
Noyon, M. & P. W. Froneman, 2013. Variability in the egg production rates of the calanoid copepod, Pseudodiaptomus hessei in a South African estuary in relation to environmental factors. Estuarine Coastal and Shelf Science 135: 306–316.
Ohman, M. D., D. L. Aksnes & J. A. Runge, 1996. The interrelationship of copepod fecundity and mortality. Limnology and Oceanography 41: 1470–1477.
Ohs, C. L., A. L. Rhyne, S. W. Grabe, M. A. DiMaggio & E. Stenn, 2010. Effects of salinity on reproduction and survival of the calanoid copepod Pseudodiaptomus pelagicus. Aquaculture 307: 219–224.
Orsi, J. & W. Mecum, 1986. Zooplankton distribution and abundance in the Sacramento-San Joaquin Delta in relation to certain environmental factors. Estuaries 9: 326–339.
Orsi, J. J. & W. L. Mecum, 1996. Food limitation as the probable cause of a long-term decline in the abundance of Neomysis mercedis the opossum shrimp in the Sacramento-San Joaquin estuary. In Hollibaugh, J. T. (ed.), San Francisco Bay: The Ecosystem. AAAS, San Francisco: 375–401.
Orsi, J. J. & S. Ohtsuka, 1999. Introduction of the Asian copepods Acartiella sinensis, Tortanus dextrilobatus (Copepoda: Calanoida), and Limnoithona tetraspina (Copepoda: Cyclopoida) to the San Francisco Estuary, California, USA. Plankton Biology and Ecology 46: 128–131.
Orsi, J. J. & T. C. Walter, 1991. Pseudodiaptomus forbesi and P. marinus (Copepoda: Calanoida), the latest copepod immigrants to California’s Sacramento-San Joaquin Estuary. In Uye, S.-I., S. Nishida & J.-S. Ho (eds) Proceedings of the fourth international conference on Copepoda. Bull. Plankton Soc. Japan, Spec. Vol., Hiroshima, 553–562.
Parker, A. E., V. E. Hogue, F. P. Wilkerson & R. C. Dugdale, 2012. The effect of inorganic nitrogen speciation on primary production in the San Francisco Estuary. Estuarine Coastal and Shelf Science 104: 91–101.
Polis, G. A., W. B. Anderson & R. D. Holt, 1997. Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annual Review of Ecology and Systematics 28: 289–316.
R Development Core Team, 2015. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
Savage, C., S. F. Thrush, A. M. Lohrer & J. E. Hewitt, 2012. Ecosystem services transcend boundaries: Estuaries provide resource subsidies and influence functional diversity in coastal benthic communities. PLoS ONE 7: e42708.
Simons, R. D., S. G. Monismith, L. E. Johnson, G. Winkler & F. J. Saucier, 2006. Zooplankton retention in the estuarine transition zone of the St Lawrence Estuary. Limnology and Oceanography 51: 2621–2631.
Skreslet, S., 1986. The role of freshwater outflow in coastal marine ecosystems, Vol. 7. Springer-Verlag, Berlin. NATO ASI Series G edn.
Slater, S. B. & R. D. Baxter, 2014. Diet, prey selection, and body condition of age-0 delta smelt, Hypomesus transpacificus, in the upper San Francisco Estuary San Francisco Estuary and Watershed Science 12: Article 1.
Slaughter, A. M., T. R. Ignoffo & W. Kimmerer, 2016. Predation impact of Acartiella sinensis, an introduced predatory copepod in the San Francisco Estuary, USA. Marine Ecology Progress Series 547: 47–60.
Sommer, T., C. Armor, R. Baxter, R. Breuer, L. Brown, M. Chotkowski, S. Culberson, F. Feyrer, M. Gingras, B. Herbold, W. Kimmerer, A. Mueller Solger, M. Nobriga & K. Souza, 2007. The collapse of pelagic fishes in the upper San Francisco Estuary. Fisheries 32: 270–277.
Sullivan, L. J. & W. J. Kimmerer, 2013. Egg development times of Eurytemora affinis and Pseudodiaptomus forbesi (Copepoda, Calanoida) from the upper San Francisco Estuary with notes on methods. Journal of Plankton Research 35: 1331–1338.
Uye, S.-I., Y. Iwai & S. Kasahara, 1983. Growth and production of the inshore marine copepod Pseudodiaptomus marinus in the central part of the Inland Sea of Japan. Marine Biology 73: 91–98.
Vuorinen, I., 1987. Vertical migration of Eurytemora (Crustacea, copepoda): a compromise between the risks of predation and decreased fecundity. Journal of Plankton Research 9: 1037–1046.
York, J. K., G. B. McManus, W. J. Kimmerer, A. M. Slaughter & T. R. Ignoffo, 2014. Trophic links in the plankton in the low salinity zone of a large temperate estuary: top-down effects of introduced copepods. Estuaries and Coasts 37: 576–588.
Acknowledgements
We thank J. Donald, R. DuMais, M. Esgro, V. Greene, A. Johnson, C. Kostecki, J. Moderan, L. Sullivan, R. Vogt, S. Westbrook, and J. Wondellock for help with field and laboratory work. M. Weaver provided helpful comments on the manuscript. Financial support came from the US Department of the Interior (RA10AC20074) and the Delta Science Program (SCI-05-C107).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Judit Padisák
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kimmerer, W.J., Ignoffo, T.R., Kayfetz, K.R. et al. Effects of freshwater flow and phytoplankton biomass on growth, reproduction, and spatial subsidies of the estuarine copepod Pseudodiaptomus forbesi . Hydrobiologia 807, 113–130 (2018). https://doi.org/10.1007/s10750-017-3385-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3385-y