Abstract
The distribution of macroinvertebrates in the heads and tails of riffles were examined in an in situ field experiment under stable baseflow conditions. Paired colonisation cylinders were used to examine the influence of vertical hydraulic exchange (upwelling and downwelling) and horizontal interstitial flow on the patterns of sedimentation and invertebrate colonisation. Sedimentation rates were greatest in cylinders permitting vertical and horizontal flow (VHE cylinders), and were significantly lower (29%) in cylinders where only vertical flow and ingress of fine sediment were possible (VE cylinders). The results demonstrate that horizontal interstitial flows represent an important pathway for fine sediment transport. Differences in fine sediment accumulation were also observed between riffle heads and tails. Significantly higher sedimentation rates were recorded in riffle tails, with the macroinvertebrate communities characterised by larger proportions of fine sediment tolerant taxa. In contrast, riffle head communities were characterised by greater proportions of sediment sensitive taxa, and in the case of VHE cylinders, shredders and EPT taxa. The results demonstrate that spatial differences in fine sediment deposition are evident at the riffle scale as a function of vertical and horizontal subsurface flows and that these factors play a key role in the distribution of macroinvertebrate fauna.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The distribution of macroinvertebrates in lotic ecosystems is typically patchy, reflecting spatial patterns which are structured by physical templates such as flow velocity (Quinn et al., 1996; Lancaster et al., 2009), substratum composition, (Dudgeon, 1982; Xu et al., 2012) and trophic processes (Culp et al., 1983; Miserendino & Masi, 2010). In addition, there is increasing recognition of the importance that vertical hydraulic and horizontal interstitial flows play in controlling these factors and their direct influence upon invertebrate communities (Dole-Oliver & Marmonier, 1992; Krause et al., 2011).
Historically there has been considerable research interest in the factors influencing the spatial distribution of benthic faunal communities from the catchment or sub-catchment scale (e.g. Rice et al., 2001; Bletter et al., 2015; Bona et al., 2015) through to the microhabitat distribution of fauna (Brooks et al., 2005; Giri et al., 2010). At intermediate scales, encompassing channel reaches and morphological units such as pool and riffle sequences, highly variable spatial distribution patterns have been reported (e.g. Logan & Brooker, 1983; Brown & Brussock, 1991; Schmera & Eros, 2011; Curry et al., 2012), although very few studies have considered the influence of the vertical hydraulic exchange or interstitial flows on macroinvertebrate composition (Hose et al., 2005; Davy-Bowker et al., 2006).
Within lowland streams and rivers, at the scale of riffle-pool sequences, water in the open channel may frequently enter the riverbed and mix/exchange with subsurface water (groundwater) within interstitial spaces. A reduction of channel depth typically forces water into the sediments at the heads of riffles resulting in downwelling water. The water passes through the interstitial spaces of the sediments (vertically and horizontally) in a downstream direction, until at the tail of the riffle, increasing water depth produces a zone of low pressure forcing water out of the sediments and into the open channel (Tonina & Buffington, 2009; Hassan et al., 2015). However, flow paths are often complex and interstitial flow may vary as a function of river stage, especially high flow events (Käser et al., 2009; Dudley-Southern & Binley, 2015). Additionally, localised areas of upwelling and downwelling water may occur as a consequence of instream biogenic features such as coarse woody debris (CWD) or macrophyte stands (Piegay & Gurnell, 1997; White & Hendricks, 2000; Lautz et al., 2006). The resulting vertical exchange of water into and out of the riverbed is spatially and temporally dynamic, leading to a mosaic of patches which are characterised by differing porosity, permeability, connectivity and physicochemical conditions (Käser et al., 2014; Sebok et al., 2015).
Vertical hydrological exchange is one of the primary controls on fine sediment deposition, storage and flushing within riverbed substrates. The accumulation of fine sediment is not uniform, reflecting the makeup of the sediment matrix, patchiness of hydraulic exchange and availability of fine sediment (Boano et al., 2007). Increases in fine sediment infiltration and deposition has the potential to reduce the porosity of the substratum, leading to a decline in vertical hydraulic flow and a reduction in the transport of organic matter, nutrients and dissolved oxygen (Bo et al., 2007; Simpson & Meixner, 2012). Substrate characteristics and hydraulic exchange have been identified as two of the most important factors driving interstitial invertebrate community composition (Brunke & Gonser, 1999; Mathers et al., 2014). Substrates characterised by high fine sediment loads are typically associated with limited hydraulic connectivity, reduced habitat quality and communities dominated by a limited pool of taxa which may feed on and/or burrow into the fine sediment deposits (Brunke, 1999; Descloux et al., 2013, 2014).
Despite significant advances in our understanding of how hydraulic exchange and associated sediment dynamics influence benthic invertebrates under laboratory conditions, (Nogaro et al., 2006; Jones et al., 2015), evidence from the field remains limited (Marmonier et al., 2010, 2012). Riffle scale variability in hydraulic exchange (upwelling, downwelling and horizontal flows) may be an important influence on the spatial distribution of benthic invertebrates (Pepin & Hauer, 2002; Capderry et al., 2013) which has received limited attention to date (Grimm et al., 2007; Krause et al., 2011). Studies which have investigated riffle scale variability in invertebrate distributions suggest that community composition differs significantly between riffle heads and tails, with both abundance and family richness being greatest at riffle heads (Pepin & Hauer, 2002; Davy-Bowker et al., 2006). Brown & Brown (1984) reported a strong correlation between some taxa with distance from riffle head, suggesting the presence of longitudinal distribution patterns within riffle units. Although there has been significant advances in understanding the structure of invertebrate communities at differing spatial scales (Mykra et al., 2007), the influence of fine sediment content and dynamics at the habitat (riffle) scale on the distribution of benthic invertebrate populations requires further elucidation though the use of field experiments (Davy-Bowker et al., 2006; Descloux et al., 2013, 2014).
This study sought to examine how fine sediment deposition varied under stable baseflow conditions between experimental colonisation cylinders subject to:
-
(a) Vertical hydraulic exchange (upwelling and downwelling);
-
(b) Vertical and horizontal interstitial hydraulic exchange
In addition the study sought to; (c) examine how the pattern of fine sediment and hydraulic exchange (upwelling, downwelling and horizontal) influenced macroinvertebrate community composition in the heads and tails of riffles.
Materials and methods
Study site
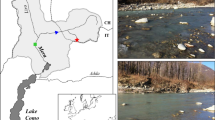
Black Brook (52°76′N,−1°32′E) is a small regulated stream located west of Loughborough (Leicestershire, UK). The river, which rises at a height of 250 m, flows into the River Soar, a tributary of the River Trent (NRFA, 2013). The catchment is underlain by Pre-Cambrian volcanic and intrusive igneous rocks covered by Triassic Mercia Mudstones and boulder clay (Greenwood et al., 2001). The river predominantly drains pastoral agricultural land before flowing through the town of Loughborough (UK). Study sites were located 800 m downstream of a small headwater reservoir (Black Brook Reservoir) to the west of Shepshed. Five riffles located within a 1200 m reach in agricultural land and which were largely shaded by deciduous trees were examined in detail during spring 2013. Hydrological data from a local gauging station on the River Soar (Kegworth, 52° 82′N,−1°27′E) indicated that the study coincided with a period of stable water levels that were not influenced by high flow events (Fig. 1).
Experimental design and invertebrate sampling
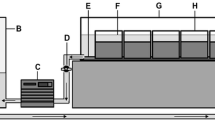
The study employed two variations of the standard colonisation cylinder for the determination of fine sediment deposition and associated invertebrate communities (Fraser et al., 1996). Open ended PVC colonisation cylinders (diameter 65 mm, height 200 mm) were assigned to one of two treatments (Fig. 2): (1) a solid PVC cylinder which allowed vertical exchange of flow (upwelling and downwelling) and fine sediment (hereafter referred to as VE cylinders–vertical exchange) or; (2) the ‘standard’ perforated colonisation cylinder design, which consisted of twelve horizontal holes (diameter 6 mm) to facilitate horizontal invertebrate colonisation (Fraser et al., 1996; Pacioglu et al., 2012; Descloux et al., 2013) and permit both horizontal and vertical exchange of flow and fine sediments (referred to as VHE cylinders–vertical and horizontal exchange). All cylinders were filled with prewashed ~1 kg of uniform size clasts (10 mm) to allow direct comparison of grains deposited within the cylinders during the experimental period and were enclosed in a net bag (7 mm aperture).
Cylinders were inserted into the river bed by threading the PVC cylinders onto a steel pipe (35 mm diameter) and driven into the bed sediments. Cylinders were inserted flush with the sediment surface to a depth of 200 mm. The surrounding stream bed remained unchanged and consisted of non-uniform cobbles and gravel. Cylinders were left in situ for 21 days, sufficient time to allow for colonisation by invertebrates and for fine sediments to accumulate (Schmid-Araya, 2000; Bo et al., 2007; Pacioglu et al., 2012). Five of each colonisation cylinder design were installed in both the riffle head and tail, providing a total of twenty colonisation samples at each riffle site (100 cylinders in total). A total of nine cylinders were lost during the experimental period reducing the total number of replicates to 91 (46 VHE cylinders and 45 VE cylinders). At the end of the experimental period, cylinders were carefully removed from the river by lifting both the PVC cylinder and net bag (containing the gravel clasts) simultaneously to minimise the loss of fines and invertebrates with a 250 µm net held directly downstream to catch any material mobilised during extraction. All invertebrate samples were preserved in the field in 10% formaldehyde and returned to the laboratory for processing and identification.
Environmental variables
Vertical hydraulic exchange was measured using mini-piezometers (Lee & Cherry, 1978) at the riffle head and tail of each site. These consisted of two open ended PVC pipes (21 mm internal diameter). The piezometer pipe comprised small perforations (two 4.5 mm holes at the base of the pipe) to enable communication with the saturated sediments and represented the water table level (Boulton, 2007). These pipes were inserted into the river bed to a depth of 200 mm (equivalent to the colonisation cylinders) by driving a stainless steel T-bar into the river bed (Boulton & Stanley, 1995; Wood et al., 2010). The top of the pipes were left protruding the water level and left to equilibrate for 24 h before use (Baxter et al., 2003). The second pipe (stage well) had solid walls and was held in the water column (at exactly the same height as the piezometer tube), but did not come into contact with the river bed, thus enabling measurement of the river stage level. The direction of vertical hydraulic exchange (vertical hydraulic gradient; VHG) was obtained through the comparison of water level depth (from the top of the pipe) via an electric dipstick [Eq. (1)]. VHG represents downwelling water if Eq. (1) produces a negative value and upwelling water if a positive value is obtained.
Laboratory procedures
In the laboratory, the contents of the colonisation cylinder samples were passed through 4 and 2 mm sieves to remove the artificial substrate. The remaining sediment was passed through a 250 µm sieve to retain invertebrates and larger clasts, with residual fine sediment (< 50 µm) collected and retained in a settling container. Material collected on all the sieves was manually processed for invertebrates. Once samples had been processed for invertebrates all grains (<2 mm) were combined with the residual fine sediment in the container and left to settle. Fine sediment samples (<2 mm) were oven dried at 60°C until a constant weight was recorded (Pacioglu et al., 2012). Samples were gently disaggregated, passed through a sieve nest (2, 1 mm and 125 µm) and each fraction weighed to determine the grain size distribution (Gordon et al., 1994). All invertebrates were identified to the lowest taxonomic level possible, usually species or genus with the exception of Oligochaeta (order), Diptera families (including Chironomidae, Tipuldae, Simuliidae and Ceratopogonidae) and Coleoptera (family).
Statistical analysis
Compositional differences in the invertebrate communities between riffle heads and tails and cylinder design were examined using non-metric multidimensional scaling (NMDS). Similarity matrices were calculated using Bray–Curtis similarity coefficients following log transformation of raw abundances (log(x + 1)). One-way Analysis of Similarities (ANOSIM) were used to test the null hypotheses H0 (no significant differences in communities at the head and tail of riffles) and H1 (no significant difference in communities between VHE and VE cylinders). Taxa contributing to the divergence of the communities were identified through the application of the similarity percentage (SIMPER) and tested for statistical differences where abundances were high enough. All multivariate analyses were performed in PRIMER software (Version 6.1.16, PRIMER-E Ltd, Plymouth, UK).
Community abundance and taxa richness data were standardised (Z-scores) prior to further analysis (Zar, 1999; Martin-Smith & Armstrong, 2002). Functional feeding traits based on Tachet et al. (2010) and abundances of taxa characterised as highly or moderately sensitive to sediment as defined by the Fine Sediment Sensitivity Ratings (FSSR given in Extence et al., 2013) were calculated for each sample. Feeding traits were assigned based on the dominant weighted group (fuzzy coded categories). Where a taxon had equal weightings for two categories, taxon abundance was assigned to both groups. In addition, individual taxon and Ephemeroptera, Plecoptera and Trichoptera (EPT) abundances were examined. Feeding trait groups, sediment sensitive macroinvertebrates, EPT and individual taxa abundances were ln(x + 1) transformed prior to analysis to comply with the underlying assumptions of the statistical tests (Gayraud et al., 2003; McMullen & Lytle, 2012).
Sediment analysis was conducted on raw grain size distribution (GSD) data. A linear mixed effects (LME) model was employed to examine grain size differences with regards to location in riffle (head or tail) and by cylinder design (VHE or VE cylinders). A LME was also employed to identify statistical differences for each of the macroinvertebrate community descriptors in the same manner. Models were fitted using the ‘nlme’ package in R Version 3.1.2 (R development Core Team, 2014). Location and cylinder design were specified as fixed factors and riffle site specified as a random factor to reflect that cylinders (both sediment deposition rates and invertebrate communities) at individual riffles are less independent then those at different riffle sites. The model was fitted using the restricted maximum likelihood (REML) estimation function.
Results
Vertical hydraulic exchange in riffle heads was downwelling at four out of five sites examined, with riffle tails characterised by upwelling water (all sites). The magnitude of vertical hydraulic exchange varied and ranged from +2.5 cm (upwelling) to −1.2 cm (downwelling), with some sites experiencing limited exchange, reflecting the stable and declining flow conditions.
Fine sediment deposition rates of riffle heads and tails
Variability in sediment deposition between riffle heads and tails
VHE cylinders More fine sediment in the 2–1 and 1 mm–125 µm fractions was deposited at the riffle tail, although there were negligible differences for the <125 µm fraction (Fig. 3a). However, GSD did not differ significantly (LME P > 0.05) between the head and tail of riffles. Average fine sediment infiltration rates were 0.00372 kg m−2 day−1.
VE cylinders There was a significantly greater volume of 1 mm–125 µm sediment deposited in riffle tails than heads (LME F 1, 39 = 5.445, P = 0.025), although differences for other size fractions were not significant (LME P > 0.05; Fig. 3b). Average fine sediment infiltration rates were 0.00264 kg m−2 day−1
Sediment deposition variability by cylinder design
Fine sediment deposition of 1–125 µm and <125 µm size fractions differed significantly between both cylinder designs in the riffle head (LME F1, 36 = 4.600, P = 0.039; and F 1, 36 = 4.770, P = 0.036) and tails (LME F 1, 42 = 10.776, P = 0.002; and F 1, 42 = 9.021, P = 0.005). In both instances, significantly greater quantities of fine sediment were deposited in the VHE cylinders (Fig. 4). VHE cylinders collected on average an additional 0.00108 kg m−2 day−1 when compared to VE cylinders.
Mean (+1 SE) infiltration rates (kg m−2 day−1) by colonisation cylinder design on Black Brook for: a riffle head and b riffle tail. Shaded = VE cylinders and open = VHE cylinders. Significant differences between the two cylinder designs (VHE and VE) for individual grain sizes are indicated by asterisks (P < 0.05; LME)
Variability in the colonisation cylinder invertebrate community between riffle heads and tails
A total of 3401 individuals were recorded in the 91 colonisation cylinders, of these 1663 occurred in the 47 VHE cylinders and 1738 in the 44 VE cylinders. A total of 32 taxa were recorded in all cylinders, of these all 32 were present within the VHE cylinders and 20 were recorded in the VE cylinders. Samples were dominated by Chironomidae (comprising multiple taxa) which accounted for 78 and 84% of the total abundance in VHE and VE cylinders, respectively. The stonefly larvae Chloroperla torrentium (Pictet, 1841) was the second most abundant taxa representing 11% of the community in VHE cylinders and 5% of the community in VE cylinders. The freshwater amphipod Gammarus pulex (Linaaeus, 1758) was the third most abundant taxa in the community representing 2% of the VHE cylinder community and 5% of the community in VE cylinders.
VHE cylinder: NMDS ordination of the VHE cylinder community (Fig. 5a) indicated no significant distinction between invertebrate community composition from the head or tail of riffles (ANOSIM R = 0.038, P = 0.12). There were no significant differences detected in community abundance or taxa richness between riffle heads and tails (LME P > 0.05). Significantly more EPT and sediment sensitive taxa were present in riffle heads (LME F 1, 29 = 6.368, P = 0.010 and F 1, 31 = 8.312, P = 0.007 respectively; Fig. 6a, b). No statistical differences were detected in scraper, predator and filterer abundances (LME P > 0.05) whilst significantly more shredders were found in riffle heads (LME F 1, 32 = 12.103, P = 0.002). The stonefly, C. torrentium was significantly more abundant in riffle heads (LME F 1, 27 = 15.888, P < 0.001; Fig. 6c). All significance values are presented in Table S1.
Non-metric multidimensional scaling (NMDS) of macroinvertebrate community data from riffle heads and tails on Black Brook: a VHE colonisation cylinder and b VE colonisation cylinder invertebrate communities (log(x + 1) transformed) based on Bray–Curtis similarity coefficients. Solid symbol = riffle head and open symbol = riffle tail
Mean (±1 SE) difference in the colonisation cylinder macroinvertebrate community metrics for riffle heads and tails on Black Brook: a EPT taxa; b sediment sensitive taxa; c C. torrentium; and d G. pulex. Diamonds and dotted line = VHE cylinders; and circles and solid line = VE cylinders. Locational differences within design are indicated by the same letter (P < 0.05, LME)
VE cylinders NMDS ordination of the VH cylinder community (Fig. 5b) indicated a significant distinction between invertebrate communities at the head and tail of riffles (ANOSIM R = 0.124, P = 0.005). There were no significant differences detected in community abundance, taxa richness or EPT abundances (Fig. 6a) between riffle heads and tails (LME P > 0.05). Significantly greater abundances of sediment sensitive taxa and shredders were determined in riffle heads (LME F 1, 32 = 5.773, P = 0.022; Fig. 5b and F 1, 1,31 = 13.546, P < 0.001), whilst there were no differences in scrapers, filterers and predators determined (LME P > 0.05). Abundances of the amphipod, G. pulex were significantly greater in riffle heads (LME F 1, 18 = 9.294, P = 0.006; Fig. 6d). All significance values are presented in Table S1.
Variability in invertebrate community by cylinder design
NMDS ordination of colonisation cylinder communities (both head and tail combined; Fig. 7a) indicated a significant distinction in the invertebrate community within VHE and VE cylinders (ANOSIM R = 0.028, P = 0.05). When location within the riffle was considered (Fig. 7b, c), significant differences in the invertebrate community within VHE and VE cylinders in the riffle head were observed (ANOSIM R = 0.124, P = 0.005) but there were no significant differences in the riffle tails (ANOSIM R = 0.016, P = 0.669).
Non-metric multidimensional scaling (NMDS) of macroinvertebrate community data (log(x + 1) transformed) from riffle heads and tails on Black Brook: by a colonisation cylinder design for all sites; b cylinder design in riffle heads; and c cylinder design in riffle tails using the Bray–Curtis similarity coefficients. Open triangles = VHE colonisation cylinders and solid triangles = VE colonisation cylinders
There were no significant differences in community abundance or taxa richness (LME P > 0.05) by cylinder design or location (head or tail). EPT taxa demonstrated significant differences by cylinder design in riffle head (LME F 1, 29 = 11.304, P = 0.002; Fig. 6a) with VHE cylinders supporting greater abundances. No significant differences were recorded for any of the functional feeding groups (LME P > 0.05). When individual taxa were considered in riffle heads, VHE cylinders supported significantly greater numbers of C. torrentium (LME F 1, 28 = 14.690, P < 0.001) whilst VE supported greater abundances of G. pulex (LME F 1, 17 = 7.317, P = 0.010; Fig. 6c, d). No significant differences by cylinder design were recorded for any of the metrics/species tested in the riffle tails (LME P > 0.05). All significance values are presented in Table S2.
Discussion
The influence of vertical and horizontal flow on sediment deposition rates
This study sought to examine the influence of vertical and horizontal flows on the deposition of fine sediment in the field. A number of studies have examined the impact of flow pathways on fine sedimentation under laboratory flume conditions (e.g. Huettel et al., 1996; Ren & Packman, 2007; Boano et al., 2014). The results of this field experiment indicate that interstitial flow (vertical and horizontal, or just vertical) exerted a strong influence on the amount of fine sediment deposition. Sediment accumulation over the experimental period was greatest in the VHE cylinders, demonstrating that subsurface flows represent an important mechanism of fine sediment transport that have been largely ignored (Petticrew et al., 2007; Rosenberry et al., 2012).
Experiments under controlled flume conditions have demonstrated that sediment traps which only allow vertical exchange (VE cylinders) reduce the trapping efficiency of fine sediment by up to 31% compared to solid-walled containers (Carling, 1984), most likely as a result of horizontal subsurface flows being disconnected. This field study recorded similar results with solid-walled containers (VE cylinders) collecting 29% less fine sediment. In contrast to ecological studies, which have employed perforated cylinders to aid invertebrate colonisation (Paciogula et al., 2012; Descloux et al., 2013), many geomorphic studies employ solid-walled containers to measure fine sediment infiltration (Beschta and Jackson 1979; Frostick et al., 1984; Wood & Armitage, 1999). Consequently, many historic studies would have probably underestimated the ingress of fines into the bed, and recent research has demonstrated that horizontal subsurface flows transport significant amounts of fine sediment (Petticrew et al., 2007). The differences in sediment accumulation between the two cylinder designs in this study indicate that the two designs can be used in parallel to provide an estimation of the relative contribution of vertical and horizontal hydraulic exchange to sediment infiltration under field conditions.
Sedimentation rates during the experimental period were greater at the tail of riffles; although with the exception of the 1 mm–125 µm fraction within the VE cylinders, differences were not statistically significant. It was anticipated that sedimentation rates would be greatest at riffle heads, due to the presence of downwelling water (Brown & Brussock, 1991; Saenger et al., 2005); however, it is likely that the stable low flow conditions (baseflow) may account for the observed patterns in this study. Infiltration rates in this study (average 0.00318 kg m−2 day−1) were lower than many other studies (Frostick et al., 1984; Sear, 1993; Wood & Armitage, 1999), reflecting the highly vegetated riparian zone of the stream (headwaters) with comparatively little direct fine sediment inputs at the study site. Despite these low rates and baseflow conditions, the role of vertical and subsurface hydrological exchange on fine sediment deposition was still clearly evident.
Cylinders were left in situ for 3 weeks and reflected the deposition patterns over the relatively short-time scale of which they were deployed. As a result, they are not suitable for estimating long-term fine sediment dynamics unless employed on multiple occasions over the full range of the hydrograph. However, this study clearly highlights the need to account for the flow regime present when interpreting results from fine sediment deposition studies. Short-term increases in discharge (over several days or a week) will result in elevated fine sediment mobilisation and consequently the results recorded will be a function of this variability. As a result, it is vitally important that the objectives of investigations which employ sediment traps are clearly established and that they are deployed under appropriate hydrological conditions in the field.
Macroinvertebrate community and taxa specific associations with vertical and horizontal exchange
This study sought to examine the influence of vertical and horizontal flows at the head and tail of riffles and the associated sediment characteristics on macroinvertebrate community composition. Only a relatively small number studies have documented differences in community characteristics at the heads and tails of riffles (Brown & Brown, 1984; Pepin & Hauer, 2002; Davy-Bowker et al., 2006). In this study, NMDS ordination indicated that cylinders which permitted only vertical hydraulic exchange (VE cylinders) supported distinct macroinvertebrate assemblages at the head and tail of riffles. In contrast, VHE cylinders supported similar communities at both the heads and tails and riffles. These subtle community differences at the riffle scale may be partially explained by the method of colonisation (Fig. 2). For VE cylinders, the method of colonisation was exclusively via vertical migration. In contrast, VHE cylinders could be colonised both horizontally and vertically resulting in greater colonisation opportunities and higher numbers of taxa being recorded.
When faunal indicator groups (EPT taxa and sediment sensitive taxa) were considered, significant differences between riffle head and tail communities were detected for both cylinder designs. Riffle tail communities were characterised by greater accumulations of fine sediment and supported less EPT taxa and sediment sensitive taxa. The composition of macroinvertebrates associated with fine sediment by location within the riffle (head or tail) indicates that fine sediment content is one of the primary factors controlling macroinvertebrate communities (Larsen et al., 2011; Wagenhoff et al., 2012).
Riffle head communities in both cylinder designs supported greater abundances of shredders, potentially as a function of the enhanced availability of organic matter (although this factor was not measured in this study; Findlay et al., 1993; Negishi & Richardson, 2003). Although the distribution of organic matter is often patchy, higher amounts of organic matter are typically found in downwelling sections of pool/riffle sequences (Pusch, 1996; Brunke & Gonser, 1999). VE cylinders subject to only vertical hydraulic exchange supported significantly greater abundances of the amphipod shredder G. pulex compared to the VHE cylinders at riffle heads. G. pulex have been widely documented to migrate vertically to utilise benthic and hyporheic sediments (Mathers et al., 2014), and may seek refuge from adverse environmental conditions and predation in subsurface habitats (McGrath et al., 2007; Wood et al., 2010). In contrast, VHE cylinders supported greater abundances of the stonefly C. torrentium. Colonisation for this species may have been aided by the presence of perforations that facilitated horizontal migration via burrowing (Hood, 1997; Xu et al., 2012). Results indicate that the use of different cylinder designs may allow the primary route and mode of colonisation for some invertebrate taxa to be determined.
Conclusion
Macroinvertebrate colonisation cylinders have witnessed a recent increase in use due to the growing implementation of in situ field experiments (Pacioglu et al., 2012; Descloux et al., 2013). Results from the study demonstrate that under stable flow conditions, differences in the faunal distribution of macroinvertebrates in riffle heads and tails were observed. Riffle head communities were characterised by a greater number of sediment sensitive taxa which reflects the patterns of sedimentation and nature of hydraulic exchange experienced during the study period. The use of two designs of colonisation cylinders also showed that horizontal subsurface flows represent an important pathway in the transport of fine sediment. The application of VE and VHE cylinders concurrently may provide a means of collecting in situ measurements which could enable the relative importance of vertical and horizontal hydraulic exchange on fine sediment dynamics (deposition and flushing) to be established in other locations. However, the study also illustrates that care is required when interpreting results derived from colonisation cylinders and sediment traps, with a clear need to contextualise the hydrological conditions during the study period (hydrograph stage). Colonisation cylinders provide an effective means of identifying the meso-scale factors driving invertebrate structures in situ, through the detailed examination of substratum, organic matter content and hydrological exchange under natural field conditions. These experiments could be further enhanced through the manipulation of fine sediment contents under a wide range of flow conditions to determine sediment infiltration rates and the conditions under which fine sediments are flushed from the bed.
References
Baxter, C., F. R. Hauer & W. W. Woessner, 2003. Measuring groundwater-stream water exchange: new techniques for installing minipiezometers and estimating hydraulic conductivity. Transactions of the American Fisheries Society 132: 493–502.
Beschta, R. L. & W. L. Jackson, 1979. The intrusion of fine sediments into a stable gravel bed. Journal of the Fisheries Research Board of Canada 36: 204–210.
Bletter, M. C. M., M. L. Amsler, I. E. de Drago, L. A. Espinola, E. Eberle, A. Paira, J. L. Best, D. R. Parsons & E. D. Drago, 2015. The impact of significant input of fine sediment on benthic fauna at tributary junctions: a case study of the Bermejo-Paraguay River confluence, Argentina. Ecohydrology 8: 340–352.
Boano, F., R. Revelli & L. Ridolfi, 2007. Bedform-induced hyporheic exchange with unsteady flows. Advances in Water Resources 20: 148–256.
Boano, F., J. W. Harvey, A. Marion, A. I. Packman, R. Revelli, L. Ridolfi & A. Wörman, 2014. Hyporheic flow and transport processes: mechanisms, models, and biogeochemical implications. Reviews of Geophysics 52: 603–679.
Bo, T., S. Fenoglio, G. Malacarna, M. Pessino & F. Sgariboldi, 2007. Effects of clogging on stream macroinvertebrates: an experimental approach. Limnologica 37: 186–192.
Bona, F., D. E. Falasco, V. L. Morgia, E. Piano, R. Ajassa & S. Fenoglio, 2015. Increased sediment loads in alpine streams: an integrated field study. River Research and Applications. doi:10.1002/rra.2941.
Boulton, A. J., 2007. Field Methods for monitoring surface/groundwater hydroecological interactions in aquatic systems. In Wood, P. J., D. M. Hannah & J. P. Sadler (eds), Hydroecology and Ecohydrology: Past, Present and Future. Wiley, Chichester.
Boulton, A. J. & E. H. Stanley, 1995. Hyporheic processes during flooding and drying in a Sonoran Desert stream II. Faunal dynamics. Archiv für Hydrobiologie 134: 27–52.
Brown, A. V. & K. B. Brown, 1984. Distribution of insects within riffles of streams. Freshwater Invertebrate Biology 3: 2–11.
Brown, A. V. & P. P. Brussock, 1991. Comparisons of benthic invertebrates between riffles and pools. Hydrobiologia 220: 99–108.
Brooks, A. J., T. Haeusler, I. Reinfields & S. Williams, 2005. Hydraulic microhabitats and the distribution of macroinvertebrate assemblages in riffles. Freshwater Biology 50: 331–344.
Brunke, M., 1999. Colmation and depth filtration within streambeds: retention of particles in hyporheic interstices. International Review of Hydrobiology 84: 99–117.
Brunke, M. & T. Gonser, 1999. Hyporheic invertebrates—the clinal nature of interstitial communities structure by hydrological exchange and environmental gradients. Journal of the North American Benthological Society 18: 344–363.
Carling, P. A., 1984. Deposition of fine and coarse sand in an open-work gravel bed. Canadian Journal of Fisheries and Aquatic Sciences 41: 263–270.
Capderry, C., T. Datry, A. Foulquier, C. Claret & F. Malard, 2013. Invertebrate distribution across nested geomorphic features in braided-river landscapes. Freshwater Science 32: 1188–1204.
Curry, C. J., R. A. Curry & D. J. Baird, 2012. The contribution of riffles and riverine wetlands to benthic macroinvertebrate biodiversity. Biodiversity Conservation 21: 895–913.
Culp, J. M., S. J. Walde & R. W. Davies, 1983. Relative importance of substrate particle size and detritus to stream benthic macroinvertebrate microdistribution. Canadian Journal of Fisheries and Aquatic Sciences 40: 1568–1574.
Davy-Bowker, J., R. W. Sweeting, N. Wright, R. T. Clarke & S. Arnott, 2006. The distribution of benthic and hyporheic macroinvertebrates from the head and tails of riffles. Hydrobiologia 563: 109–123.
Descloux, S., T. Datry & P. Marmonier, 2013. Benthic and hyporheic invertebrate assemblages along a gradient of increasing streambed colmation by fine sediment. Aquatic Sciences 75: 493–507.
Descloux, S., T. Datry & P. Marmonier, 2014. Trait-based structure of invertebrates along a gradient of sediment colmation with fine sediments. International Review of Hydrobiology 95: 520–540.
Dole-Oliver, M.-J. & P. Marmonier, 1992. Patch distribution of interstitial communities: prevailing factors. Freshwater Biology 27: 177–191.
Dudgeon, D., 1982. Aspects of the microdistribution of insect macrobenthos in a forest stream in Hong Kong. Archiv Hydrobiolgia Suppl 64: 221–239.
Dudley-Southern, M. & A. Binley, 2015. Temporal responses of groundwater-surface water exchange to successive storm events. Water Resources Research 51: 112–1126.
Extence, C. A., R. P. Chadd, J. England, M. J. Dunbar, P. J. Wood & E. D. Taylor, 2013. The assessment of fine sediment accumulation in rivers using macro-invertebrate community response. River Research and Applications 29: 17–55.
Findlay, S., D. Strayer, C. Goumbla & K. Gould, 1993. Metabolism of streamwater dissolved organic carbon in the shallow hyporheic zone. Limnology and Oceanography 38: 1493–1499.
Fraser, B. G., D. D. Williams & K. W. F. Howard, 1996. Monitoring biotic and abiotic processes across the hyporheic/groundwater interface. Hydrogeology 4: 36–50.
Frostick, L. E., P. M. Lucas & I. Reid, 1984. The infiltration of fine matrices into coarse-grained alluvial sediments and its implications for stratigraphic interpretation. Journal of the Geological Society of London 141: 955–965.
Gayraud, S., B. Statzner, P. Bady, A. Haybachip, F. Scholl, P. Usseglo-Polatera & M. Bacchi, 2003. Invertebrate traits for the biomonitoring of large European rivers: an initial assessment of alternative metrics. Freshwater Biology 48: 2045–2064.
Giri, M. L., E. T. Chester & B. J. Robson, 2010. Does sampling method or microhabitat type determine patterns of macroinvertebrate assemblage structure detected across spatial scales in rivers? Marine and Freshwater Research 61: 1313–1317.
Gordon, N. D., T. A. McMahon & B. L. Finlayson, 1994. Stream Hydrology: An Introduction for Ecologists. Wiley, Chichester.
Greenwood, M. T., M. A. Bickerton & G. E. Petts, 2001. Assessing adult Trichoptera communities of small streams: a case study from Charnwood Forest, Leicestershire, UK. Aquatic Conservation: Marine and Freshwater Ecosystems 11: 93–107.
Grimm, N. B., C. V. Baxter & C. L. Crenshaw, 2007. Surface-subsurface interactions in streams. In Haeur, F. R. & G. A. Lamberti (eds), Methods in Stream Ecology. Academic press, San Diego.
Hassan, M. A., D. Tonina, R. D. Beckie & M. Kinnear, 2015. The effects of discharge and slope on hyporheic flow in step-pool morphologies. Hydrological Processes 29: 419–433.
Hood, R., 1997. Vertical, longitudinal and seasonal variation in benthic community structure in a fourth order stream ecosystem. The American Midland Naturalist 146: 223–242.
Hose, G. C., P. Jones & R. P. Lim, 2005. Hyporheic macroinvertebrates in riffle and pool areas of temporary streams in south eastern Australia. Hydrobiologia 532: 81–90.
Huettel, M., W. Ziebis & S. Forster, 1996. Flow-induced uptake of particulate matter in permeable sediments. Limnology and Oceanography 4: 309–322.
Jones, I., I. Growns, A. Arnold, S. McCall & M. Bowes, 2015. The effect of increased flow and fine sediment on hyporheic invertebrates and nutrients in stream mesocosms. Freshwater Biology 60: 813–826.
Käser, D. H., A. Binley, A. L. Heathwaite & S. Krause, 2009. Spatio-temporal variations of hyporheic flow in a riffle-step-pool sequence. Hydrological Processes 23: 2138–2149.
Käser, D. H., A. Binley, S. Krause & A. L. Heathwaite, 2014. Prospective modelling of 3D hyporheic exchange based on high-resolution topography and stream elevation. Hydrological Processes 28: 2579–2594.
Krause, S., D. M. Hannah, J. H. Fleckenstein, C. M. Heppell, D. Kaeser, R. Pickup, G. Pinay, A. L. Robertson & P. J. Wood, 2011. Inter-disciplinary perspectives on processes in the hyporheic zone. Ecohydrology 4: 481–499.
Lancaster, J., B. J. Downes & A. Glaister, 2009. Interacting environmental gradients, trade-offs and reversals in the abundance-environment relationships of stream insects: when flow is unimportant. Marine and Freshwater Research 60: 259–270.
Larsen, S., G. Pace & S. J. Ormerod, 2011. Experimental effects of sediment deposition on the structure and function of macroinvertebrate assemblages in temperate streams. River Research and Applications 27: 257–267.
Lautz, L. K., D. I. Siegel & R. L. Bauer, 2006. Impact of debris dams on hyporheic interaction along a semi-arid stream. Hydrological Processes 20: 183–196.
Lee, D. R. & J. A. Cherry, 1978. A field exercise on groundwater flow using seepage meters and min-piezometers. Journal of Geological Education. 27: 6–10.
Logan, P. & M. P. Brooker, 1983. The macroinvertebrate fauna of riffles and pools. Water Research 17: 263–270.
McGrath, K. E., E. T. H. M. Peeters, J. A. Beijer & M. Scheffer, 2007. Habitat-mediated cannibalism and microhabitat restriction in the stream invertebrate Gammarus pulex. Hydrobiologia 589: 155–164.
Mathers, K. L., J. Millett, A. L. Robertson, R. Stubbington & P. J. Wood, 2014. Faunal response to benthic and hyporheic sedimentation varies with direction of vertical hydrological exchange. Freshwater Biology 59: 2278–2289.
Marmonier, P., H. Luczyszyn, M. Creuze des Chatelliers, N. Landon, C. Claret & M. -J. Dole-Olivier, 2010. Hyporheic flowpaths and interstitial invertebrates associated with stable and eroded river sections: interactions between micro and meso-scales. Fundamental and Applied Limnology 176: 303–317.
Marmonier, P., G. Archambaud, N. Belaidi, N. Bougon, P. Breil, E. Chauvet, C. Claret, J. Cornut, T. Datry, M.-J. Dole-Olivier, B. Dumont, N. Flipo, A. Foulquier, M. Gerino, A. Guilpart, F. Julien, C. Maazouzi, D. Martin, F. Mermillod-Blondin, B. Montuelle, Ph Namour, S. Navel, D. Ombredane, T. Pelte, C. Piscart, M. Pusch, S. Stroffek, A. Robertson, J.-M. Sanchez-Perez, S. Sauvage, A. Taleb, M. Wantzen & Ph Vervier, 2012. The role of organism in hyporheic processes: gaps in current knowledge, needs for future research and applications. Annales de Limnologie: International Journal of Limnology 48: 253–266.
Martin-Smith, K. M. & J. D. Armstrong, 2002. Growth rates of wild stream-dwelling Atlantic salmon correlate with activity and sex but not dominance. Journal of Animal Ecology 71: 413–423.
McMullen, L. E. & D. A. Lytle, 2012. Quantifying invertebrate resistance to flood: a global-scale meta-analysis. Ecological Applications 22: 2164–2175.
Miserendino, M. L. & C. I. Masi, 2010. The effects of land use on environmental fetrues and functional organization of macroinvertebrate communities in Patagonian low order streams. Ecological Indicators 10: 311–319.
Mykra, H., J. Heino & T. Muotka, 2007. Scale related patterns in the spatial environmental compoonents of stream macroinvebrate assemblage variation. Global Ecology and Biogeography 16: 149–159.
National River Flow Archive, 2013. Black Brook at One Barrow Data Webpage. Available at: http://www.ceh.ac.uk/data/nrfa/data/station.html?28030. [Access Date: 19 Dec 2015].
Negishi, J. N. & J. S. Richardson, 2003. Responses of organic matter and macroinvertebrates to placements of boulder clusters in a small stream of southwestern British Columbia, Canada. Canadian Journal of Fisheries and Aquatic Science 60: 247–258.
Nogaro, G., F. Mermillod-Blondin, F. Francois-Carcaillet, J.-P. Gaudet, M. Lafont & J. Gibert, 2006. Invertebrate bioturbation can reduce clogging of sediment: an experimental study using infiltration sediment columns. Freshwater Biology 51: 1458–1473.
Pacioglu, O., P. Shaw & A. Robertson, 2012. Patch scale response of hyporheic invertebrates to fine sediment removal in two chalk rivers. Fundamental and Applied Limnology 18: 283–288.
Pepin, D. M. & F. R. Hauer, 2002. Benthic response to groundwater-surface water exchange in 2 alluvial rivers in north-western Montana. Journal of the North American Benthological Society 21: 370–383.
Petticrew, E. L., A. Krein & D. E. Walling, 2007. Evaluating fine sediment mobilization and storage in a gravel-bed river using controlled reservoir releases. Hydrological Processes 21: 198–210.
Piegay, H. & A. M. Gurnell, 1997. Large woody debris and river geomorphological pattern: examples from S.E. France and S. England. Geomorphology 19: 99–116.
Pusch, M., 1996. The metabolism of organic matter in the hyporheic zone of a mountain stream, and its spatial distribution. Hydrobiologia 323: 107–118.
Quinn, J. M., C. W. Hickey & W. Linklater, 1996. Hydraulic influences on periphyton and benthic macroinvertebrates: simulating the effects of upstream bed roughness. Freshwater Biology 35: 301–309.
Development Core Team, R., 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0.
Ren, J. & A. I. Packman, 2007. Changes in fine sediment size distributions due to interactions with streambed sediments. Sediment Geology 3: 529–537.
Rice, S. P., M. T. Greenwood & C. B. Joyce, 2001. Macroinvertebrate community changes at coarse sediment recruitment points along two gravel-bed rivers. Water Resources Research 37: 2793–2803.
Rosenberry, D. O., P. Zion Klos & A. Neal, 2012. In situ quantification of spatial and temporal variability of hyporheic exchange in static and mobile gravel-bed rivers. Hydrological Processes 26: 604–612.
Saenger, N., P. K. Kitandis & R. L. Street, 2005. A numerical study of surface-subsurface exchange processes at a riffle-pool pair in the Lahn River, Germany. Water Resources Research 41: W12424.
Schmera, D. & T. Eros, 2011. The role of sampling effort, taxonomical resolution and abundance weight in multivariate comparison of stream dwelling caddisfly assemblages collected from riffle and pool habitats. Ecological Indicators 11: 230–239.
Schmid-Araya, J. M., 2000. Invertebrate recolonisation patterns in the hyporheic zone of a gravel stream. Limnology and Oceanography 45: 1000–1005.
Sear, D. A., 1993. Fine sediment infiltration into gravel spawning beds within a regulated river experiencing floods: ecological implications for salmonids. Regulated Rivers: Research and Management 8: 373–390.
Sebok, E., C. Duque, P. Engesgaard & E. Boegh, 2015. Spatial variability in streambed hydraulic conductivity of contrasting stream morphologies: channel bend and straight channel. Hydrological Processes 29: 458–472.
Simpson, S. C. & T. Meixner, 2012. Modelling effects of floods on streambed hydraulic conductivity and groundwater–surface water interactions. Water Resources Research 48: W02515.
Tachet, H., M. Bournaud, P. Richoux & P. Usseglio-Polatera, 2010. Invertébrés d’eau douce : Systématique, Biologie, Écologie. CNRS Editions, Paris.
Tonina, D. & J. M. Buffington, 2009. Hyporheic exchange in Mountain Rivers: mechanics and environment effects. Geography Compass 3: 1053–1086.
Wagenhoff, A., C. R. Townsend & C. D. Matthaei, 2012. Macroinvertebrate responses along broad stressor gradients of deposited fine sediment and dissolved nutrients: a stream mesocosm experiment. Journal of Applied Ecology 49: 892–902.
White, D. S. & S. P. Hendricks, 2000. Lotic macrophytes and surface-subsurface exchange processes. In Jones, J. B. & P. J. Mulholland (eds), Streams and Ground Waters. Academic Press, London: 363–379.
Wood, P. J. & P. D. Armitage, 1999. Sediment deposition in a small lowland stream-management implications. Regulated Rivers: Research and Management 15: 199–210.
Wood, P. J., A. J. Boulton, S. Little & R. Stubbington, 2010. Is the hyporheic zone a refugium for macroinvertebrates during severe low flow conditions? Fundamental and Applied Limnology 176: 377–390.
Xu, M. Z., Z. Y. Wang, B. Z. Pan & N. Zhao, 2012. Distribution and species composition of macroinvertebrates in the hyporheic zone of bed sediment. International Journal of Sediment Research 27: 129–140.
Zar, J. H., 1999. Biostatistical Analysis, 4th ed. Prentice Hall, Englewood Cliffs.
Acknowledgments
KLM acknowledges the Department of Geography Laboratory staff, Stuart Ashby and Barry Kenny, for technical and laboratory support. Thanks go to Joni Cook who provided assistance with fieldwork. The Environment Agency is gratefully acknowledged for providing river stage data. The authors thank the two anonymous reviewers for their helpful and constructive comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Marcelo S. Moretti
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mathers, K.L., Wood, P.J. Fine sediment deposition and interstitial flow effects on macroinvertebrate community composition within riffle heads and tails. Hydrobiologia 776, 147–160 (2016). https://doi.org/10.1007/s10750-016-2748-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2748-0