Abstract
Climate change may affect species diversity and, consequently, ecological processes such as leaf decomposition. We evaluated the effects of increased temperature and carbon dioxide (CO2) on fungal biomass, leaf breakdown, and on survival and growth of the shredder Phylloicus elektoros. We hypothesized that climatic changes would result in lower survival and growth of shredders and lower leaf consumption by these organisms. On the other hand, we predicted an increase in fungal biomass in response to climatic changes. We conducted an experiment in Manaus, Brazil, using four microcosms that simulate real-time air temperature and CO2 (control chamber), as well as three other chambers subjected to fixed increases in temperature and CO2 as compared to the control chamber. The “extreme” condition represented an increase of ~4.5°C in temperature and ~870 ppm in CO2 in relation to the control chamber. Total and shredder leaf-breakdown rates, fungal biomass, and shredder survival rates were significantly lower in warmer and CO2 concentrated atmospheres. Shredder growth rate and leaf breakdown by microorganisms were similar among all climatic conditions. With climatic changes, we found an increase in the relative importance of microorganisms on leaf-breakdown rates as compared to shredders. Thus, lower leaf breakdown and a change in the main decomposer due to future climatic conditions may result in major changes in the pathways of organic matter processing and, consequently, in aquatic food webs.





Similar content being viewed by others
References
Abelho, M., 2001. From litterfall to breakdown in streams: a review. The Scientific World 1: 656–680.
Abelho, M., 2009. ATP and ergosterol as indicators of fungal biomass during leaf decomposition in streams: a comparative study. International Review of Hydrobiology 94: 3–15.
Adams, J. A., N. C. Tuchman & P. A. Moore, 2003. Atmospheric CO2 enrichment alters leaf detritus: impacts on foraging decisions of crayfish (Orconectes virilis). Journal of the North American Benthological Society 22: 410–422.
Albariño, R. J. & E. G. Balseiro, 2001. Food Quality, Larval consumption, and growth of Klapopteryx kuscheli (Plecoptera: Austroperlidae) from a South Andes stream. Journal of Freshwater Ecology 16: 517–526.
Altman, D. G. & J. M. Bland, 1998. Time to event (survival) data. British Medical Journal 317: 468–469.
Anderson, N. H. & K. W. Cummins, 1979. Influences of diet on the life histories of aquatic insects. Journal of the Fisheries Research Board of Canada 36: 335–342.
Anderson, N. H. & E. Grafius, 1975. Utilization and processing of allochthonous material by stream Trichoptera. Verhandlungen des Internationalen Verein Limnologie 19: 3083–3088.
Ardón, N., L. A. Stallcup & C. M. Pringle, 2006. Does leaf quality mediate the stimulation of leaf breakdown by phosphorus in Neotropical streams? Freshwater Biology 51: 618–633.
Atkinson, D. & R. M. Sibly, 1997. Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends in Ecology and Evolution 12: 235–239.
Bärlocher, F. & M. A. S. Graça, 2005. Total phenolics. In Graça, M. A. S., F. Bärlocher & M. O. Gessner (eds), Methods to Study Litter Decomposition: A Practical Guide. Springer, Dordrecht: 97–100.
Bastian, M., L. Boyero, B. R. Jackes & R. G. Pearson, 2007. Leaf litter diversity and shredder preferences in an Australian tropical rain-forest stream. Journal of Tropical Ecology 23: 219–229.
Becker, B., M. S. Moretti & M. Callisto, 2009. Length–dry mass relationships for a typical shredder in Brazilian streams (Trichoptera: Calamoceratidae). Aquatic Insects 31: 227–234.
Bisutti, I., I. Hilke & M. Raessler, 2004. Determination of total organic carbon – an overview of current methods. Trends in Analytical Chemistry 23: 716–726.
Bland, J. M., 2004. The logrank test. British Medical Journal 328: 1073.
Bland, J. M. & D. G. Altman, 1998. Survival probabilities (the Kaplan–Meier method). British Medical Journal 317: 1572.
Boyero, L., R. G. Pearson, M. O. Gessner, L. A. Barmuta, V. Ferreira, M. A. S. Graça, D. Dudgeon, A. J. Boulton, M. Callisto, E. Chauvet, J. E. Helson, A. Bruder, R. J. Albariño, C. M. Yule, M. Arunachalam, J. N. Davies, R. Figueroa, A. S. Flecker, A. Ramírez, R. G. Death, T. Iwata, J. M. Mathooko, C. Mathuriau, J. F. Gonçalves Jr, M. S. Moretti, T. Jinggut, S. Lamothe, C. M’Erimba, L. Ratnarajah, M. H. Schindler, J. Castela, L. M. Buria, A. Cornejo, V. D. Villanueva & D. C. West, 2011. A global experiment suggests climate warming will not accelerate litter decomposition in streams but might reduce carbon sequestration. Ecology Letters 14: 289–294.
Boyero, L., B. J. Cardinale, M. Bastian & R. G. Pearson, 2014. Biotic versus abiotic control of decomposition: a comparison of the effects of simulated extinctions and changes in temperature. PLoS One 9: e87426.
Canadell, J. G., C. Quéré, M. R. Raupach, C. B. Field, E. T. Buitenhuis, P. Ciais, T. J. Conway, N. P. Gillett, R. A. Houghton & G. Marland, 2007. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proceedings of the National Academy of Sciences 104: 18866–18870.
Canhoto, C. & M. A. S. Graça, 1995. Food value of introduced eucalypt leaves for a Mediterranean stream detritivore: Tipula lateralis. Freshwater Biology 34: 209–214.
Carvalho, E. M. & M. A. S. Graça, 2007. A laboratory study on feeding plasticity of the shredder Sericostoma vittatum Rambur (Sericostomatidae). Hydrobiologia 575: 353–359.
Chauvet, E. & K. Suberkropp, 1998. Temperature and sporulation of aquatic hyphomycetes. Applied and Environmental Microbiology 64: 1522–1525.
Coviella, C. E. & J. T. Trumble, 1999. Effects of elevated atmospheric carbon dioxide on insect–plant interactions. Conservation Biology 13: 700–712.
Cummins, K. W. & M. J. Klug, 1979. Feeding ecology of stream invertebrates. Annual Review of Ecology and Systematics 10: 147–172.
Cummins, K. W., R. C. Petersen, F. O. Howard, J. C. Wuycheck & V. I. Holt, 1973. The utilization of leaf litter by stream detritivores. Ecology 54: 336–345.
Davies, J. N. & A. J. Boulton, 2009. Great house, poor food: effects of exotic leaf litter on shredder densities and caddisfly growth in 6 subtropical Australian streams. Journal of the North American Benthological Society 28: 491–503.
Feely, R. A., S. C. Doney & S. R. Cooley, 2009. Ocean acidification: present conditions and future changes in a high-CO2 world. Oceanography 22: 36–47.
Fernandes, I., B. Uzun, C. Pascoal & F. Cássio, 2009. Responses of aquatic fungal communities on leaf litter to temperature-change events. International Review of Hydrobiology 94: 410–418.
Ferreira, V. & E. Chauvet, 2011a. Future increase in temperature more than decrease in litter quality can affect microbial litter decomposition in streams. Oecologia 167: 279–291.
Ferreira, V. & E. Chauvet, 2011b. Synergistic effects of water temperature and dissolved nutrients on litter decomposition and associated fungi. Global Change Biology 17: 551–564.
Ferreira, V., A. Gonçalves, D. L. Godbold & C. Canhoto, 2010. Effect of increased atmospheric CO2 on the performance of an aquatic detritivore through changes in water temperature and litter quality. Global Change Biology 16: 3284–3296.
Ferreira, V., A. C. Encalada & M. A. S. Graça, 2012. Effects of litter diversity on decomposition and biological colonization of submerged litter in temperate and tropical streams. Freshwater Science 31: 945–962.
Foucreau, N., S. Puijalon, F. Hervant & C. Piscart, 2013. Effect of leaf litter characteristics on leaf conditioning and on consumption by Gammarus pulex. Freshwater Biology 58: 1672–1681.
Friberg, N. & D. Jacobsen, 1994. Feeding plasticity of two detritivore-shredders. Freshwater Biology 32: 133–142.
Friberg, N. & D. Jacobsen, 1999. Variation in growth of the detritivore-shredder Sericostoma personatum (Trichoptera). Freshwater Biology 42: 625–635.
Geraldes, P., C. Pascoal & F. Cássio, 2012. Effects of increased temperature and aquatic fungal diversity on litter decomposition. Fungal Ecology 5: 734–740.
Gessner, M. O., 2005. Proximate lignin and cellulose. In Graça, M. A. S., F. Bärlocher & M. O. Gessner (eds), Methods to Study Litter Decomposition: A Practical Guide. Springer, Dordrecht: 115–120.
Gessner, M. O. & E. Chauvet, 1994. Importance of stream microfungi in controlling breakdown rates of leaf-litter. Ecology 75: 1807–1817.
Giberson, D. J. & D. M. Rosenberg, 1992. Effects of temperature, food quantity, and nymphal rearing density on life-history traits of a northern population of Hexagenia (Ephemeroptera: Ephemeridae). Journal of the North American Benthological Society 11: 181–193.
Gonçalves, A. L., M. A. S. Graça & C. Canhoto, 2013. The effect of temperature on leaf decomposition and diversity of associated aquatic hyphomycetes depends on the substrate. Fungal Ecology 6: 546–553.
Gonçalves, J. F., J. S. França, A. O. Medeiros, C. A. Rosa & M. Callisto, 2006. Leaf breakdown in a tropical stream. International Review of Hydrobiology 91: 164–177.
Graça, M. A. S., 2001. The role of invertebrates on leaf litter decomposition in stream: a review. International Review of Hydrobiology 86: 383–393.
Graça, M. A. S. & F. Bärlocher, 2005. Radial diffusion assay for s. In Graça, M. A. S., F. Bärlocher & M. O. Gessner (eds), Methods to Study Litter Decomposition: A Practical Guide. Springer, Dordrecht: 101–107.
Graça, M. A. S. & C. Cressa, 2010. Leaf quality of some tropical and temperate tree species as food resource for stream shredders. International Review Hydrobiology 1: 27–41.
Graça, M. A. S. & M. Zimmer, 2005. Leaf toughness. In Graça, M. A. S., F. Bärlocher & M. O. Gessner (eds), Methods to Study Litter Decomposition: A Practical Guide. Springer, Dordrecht: 121–128.
Graça, M. A. S., C. Cressa, M. O. Gessner, M. J. Feio, K. A. Callies & C. Barrios, 2001. Food quality, feeding preferences, survival and growth of shredders from temperate and tropical streams. Freshwater Biology 46: 947–957.
Grafius, E. & N. H. Anderson, 1979. Population dynamics, bioenergetics, and role of Lepidostoma quercina Ross (Trichoptera: Lepidostomatidae) in an Oregon woodland stream. Ecology 60: 433–441.
Grafius, E. & N. H. Anderson, 1980. Population dynamics and role of two species of Lepidostoma (Trichoptera: Lepidostomatidae) in an Oregon coniferous forest stream. Ecology 61: 808–816.
Hurlbert, S. H., 1984. Pseudoreplication and the design of ecological field experiments. Ecological monographs 54: 187–211.
IPCC – Intergovernmental Panel on Climate Change, 2007. Climate Change 2007: The Physical Science Basis. Contribution of the Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge.
Iversen, T. M., 1979. Laboratory Energetics of Larvae of Sericostoma personatum (Trichoptera). Holarctic Ecology 2: 1–5.
Johns, C. V. & L. Hughes, 2002. Interactive effects of elevated CO2 and temperature on the leaf-miner Dialectica scalariella Zeller (Lepidoptera: Gracillariidae) in Paterson’s Curse, Echium plantagineum (Boraginaceae). Global Change Biology 8: 142–152.
Kingsolver, J. G. & R. B. Huey, 2008. Size, temperature, and fitness: three rules. Evolutionary Ecology Research 10: 251–268.
Kominoski, J. S. & A. D. Rosemond, 2012. Conservation from the bottom up: forecasting effects of global change on dynamics of organic matter and management needs for river networks. Freshwater Science 31: 51–68.
Landeiro, V. L., N. Hamada & A. S. Melo, 2008. Responses of aquatic invertebrate assemblages and leaf breakdown to macroconsumer exclusion in Amazonian “terra firme” streams. Fundamental and Applied Limnology 172: 49–58.
Landeiro, V. L., N. Hamada, B. S. Godoy & A. S. Melo, 2010. Effects of litter patch area on macroinvertebrate assemblage structure and leaf breakdown in Central Amazonian streams. Hydrobiologia 649: 355–363.
Li, A. O. Y. & D. Dudgeon, 2008. The effects of leaf litter characteristics on feeding and fitness of a tropical stream shredder, Anisocentropus maculatus (Trichoptera: Calamoceratidae). Marine and Freshwater Research 59: 897–901.
Martins, R. T., A. S. Melo, J. F. Gonçalves & N. Hamada, 2014. Estimation of dry mass of caddisflies Phylloicus elektoros (Trichoptera: Calamoceratidae) in a Central Amazon stream. Zoologia 31: 337–342.
Martins, R. T., A. S. Melo, J. F. G. Júnior & N. Hamada, 2015. Leaf-litter breakdown in urban streams of Central Amazonia: direct and indirect effects of physical, chemical, and biological factors. Freshwater Science 34: e10.1086/681086.
Mas-Martí, E., A. M. Romaní & I. Muñoz, 2015. Consequences of warming and resource quality on the stoichiometry and nutrient cycling of a stream shredder. PLoS One 10: e0118520.
Moline, A. B. & N. L. Poff, 2008. Growth of an invertebrate shredder on native (Populus) and non-native (Tamarix, Elaeagnus) leaf litter. Freshwater Biology 53: 1012–1020.
Mooney, H., A. Larigauderie, M. Cesario, T. Elmquist, O. Hoegh-Guldberg, S. Lavorel, G. M. Mace, M. Palmer, R. Scholes & T. Yahara, 2009. Biodiversity, climate change, and ecosystem services. Current Opinion in Environmental Sustainability 1: 46–54.
Moretti, M. S., R. Loyola, B. Becker & M. Callisto, 2009. Leaf abundance and phenolic concentrations codetermine the selection of case-building materials by Phylloicus sp. (Trichoptera, Calamoceratidae). Hydrobiologia 630: 199–206.
Navarro, F. K. S. P., R. S. Rezende & J. F. Gonçalves, 2013. Experimental assessment of temperature increase and presence of predator carcass changing the response of invertebrate shredders. Biota Neotropica 13: 28–33.
Nolen, J. A. & R. G. Pearson, 1993. Factors affecting litter processing by Anisocentropus kirramus (Trichoptera: Calamoceratidae) from an Australian tropical rainforest stream. Freshwater Biology 29: 469–479.
Novozamsky, J., V. J. G. Houba, R. van Eck & W. van Vark, 1983. A novel digestion technique for multielement plant analysis. Communications in Soil Science and Plant Analysis 14: 239–248.
Park, S. & K. H. Cho, 2003. Nutrient leaching from leaf litter of emergent macrophyte (Zizania latifolia) and the effects of water temperature on the leaching process. Korean Journal of Biological Sciences 7: 289–294.
Petchey, O. L., P. T. McPhearson, T. M. Casey & P. J. Morin, 1999. Environmental warming alters food-web structure and ecosystem function. Nature 402: 69–72.
Peterson, C. H. & P. E. Renaud, 1989. Analysis of feeding preference experiments. Oecologia 80: 82–86.
Prather, A. L., 2003. Revision of the Neotropical caddisfly genus Phylloicus (Trichoptera: Calamoceratidae). Zootaxa 275: 1–214.
Rajashekhar, M. & K. M. Kaveriappa, 2000. Effects of temperature and light on growth and sporulation of aquatic hyphomycetes. Hydrobiologia 441: 149–153.
Rincón, J. & I. Martínez, 2006. Food quality and feeding preferences of Phylloicus sp. (Trichoptera:Calamoceratidae). Journal of the North American Benthological Society 25: 209–215.
Rumbos, C. I., D. Stamopoulos, G. Georgoulas & E. Nikolopoulou, 2010. Factors affecting leaf litter decomposition by Micropterna sequax (Trichoptera: Limnephilidae). International Review of Hydrobiology 95: 383–394.
Sridhar, K. R. & F. Bärlocher, 1993. Effect of temperature on growth and survival of five aquatic hyphomycetes. Sydowia 45: 377–387.
Swan, C. M. & M. A. Palmer, 2006. Composition of speciose leaf litter alters stream detritivore growth, feeding activity and leaf breakdown. Oecologia 147: 469–478.
Sweeney, B. W. & R. L. Vannote, 1978. Size variation and distribution of hemimetabolous aquatic insects: two thermal equilibrium hypotheses. Science 200: 444–446.
Tuchman, N. C., K. A. Wahtera, R. G. Wetzel & J. A. Teeri, 2003. Elevated atmospheric CO2 alters leaf litter quality for stream ecosystems: an in situ leaf decomposition study. Hydrobiologia 495: 203–211.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137.
Verberk, W. C. E. P. & D. T. Bilton, 2013. Respiratory control in aquatic insects dictates their vulnerability to global warming. Biology Letters 9: 1–4.
Villanueva, V. D., R. Albariño & C. Canhoto, 2011. Detritivores feeding on poor quality food are more sensitive to increased temperatures. Hydrobiologia 678: 155–165.
Wagner, R., 1990. Influence of temperature, photoperiod and nutrition on growth and consumption of Chaetopteryx villosa (Trichoptera). Holarctic Ecology 13: 247–254.
Waldbauer, G. P., 1968. The consumption and utilization of food by insects. Advances in Insect Physiology 5: 229–288.
Witkowski, E. T. F. & B. B. Lamont, 1991. Leaf specific mass confounds leaf density and thickness. Oecologia 88: 486–493.
Acknowledgments
We thank Dr. Adalberto L. Val for microcosm use, Dr. Ana M.O. Pes for Phylloicus elektoros identification, Dr. Sérgio Nunomura for lyophilizer use, Dr. Manuel A.S. Graça for suggestions during data analysis, and Fernanda Dragan and Jéssica Oliveira for help during the experiment. We also thank Fernanda Dragan and collaborators who are finalizing a detailed description of the microcosms. ASM, JFGJr, and NH received research fellowships (procs. 307479/2011-0, 302957/2014-6 and 306328/2010-0, respectively) from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). RTM received a fellowship from Programa de Apoio à Fixação de Doutores no Amazonas—FIXAM/AM. CT-Amazônia/CNPq (Proc. 575875/2008-9), Pronex/CNPq/Fapeam—Aquatic insects, CT-Hidro/Climatic Changes/Water Resources/CNPq (Proc. 403949/2013-0) and INCT/ADAPTA (CNPq/FAPEAM)—Amazon projects supported the invertebrate sample collection, laboratory analyses, and microcosm experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Adalberto L. Val, Gudrun De Boeck, & Sidinei M. Thomaz / Adaptation of Aquatic Biota of the Amazon
Electronic supplementary material
Below is the link to the electronic supplementary material.
10750_2016_2689_MOESM1_ESM.pdf
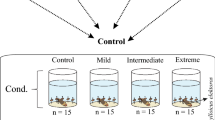
Supplementary material 1 (PDF 496 kb). Fig. S1—Flow diagram of the experimental design. We considered total leaf breakdown as sum of microbial leaf breakdown and shredder leaf breakdown. The values of air temperature and CO2 indicated are the averages registered during the experiment. Chambers were subjected to the following conditions: Control: real-time current conditions of air temperature and CO2 from Manaus (Amazonas, Brazil); Light: increases of ~1.5°C in temperature and ~220 ppm CO2 concentration in relation to the control; Intermediate: increases of ~3.0°C in temperature and ~420 ppm CO2 concentration in relation to the control; Extreme: increase of ~4.5°C in temperature and ~870 ppm CO2 concentration in relation to the control
Rights and permissions
About this article
Cite this article
Martins, R.T., Melo, A.S., Gonçalves, J.F. et al. Effects of climate change on leaf breakdown by microorganisms and the shredder Phylloicus elektoros (Trichoptera: Calamoceratidae). Hydrobiologia 789, 31–44 (2017). https://doi.org/10.1007/s10750-016-2689-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2689-7