Abstract
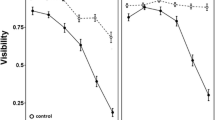
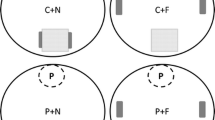
Food acquisition by most organisms is a complex ecological process that involves benefits and risks, affecting organism development and interspecific interactions. The evaluation of habitat selection, food consumption, and predator avoidance is pivotal for understanding the ecological process affecting life history traits and the role of species on communities and ecosystems. In a microcosm experiment, we evaluated if Rhinella diptycha tadpoles actively choose to forage in habitats with high resource (food) availability and if they avoid such habitats when predators are positively correlated with resource distribution. We also evaluated if behavioral changes under predation risk were associated with specific morphological phenotypes. We observed that tadpoles chose, although not intensely, habitats with high resource availability when predator cues were absent, but they avoided the same habitats when predation cues were present. We also observed an increase in swimming activity and morphological changes in tadpoles exposed to predation risk, especially related to body and tail morphology, which translates into rapid development. Our results suggest that tadpoles assess habitat quality through resource availability and predation risk. Moreover, our results suggest that tadpoles seem to exhibit functionally independent co-specialization of defensive strategies, due to the expression of specific behavioral and morphological phenotypes.




Similar content being viewed by others
References
Abrahams, M. & L. Dill, 1989. A determination of the energetic equivalence of the risk of predation. Ecology 70: 999–1007.
Alonzo, S. H., 2002. State-dependent habitat selection games between predators and prey: the importance of behavioural interactions and expected lifetime reproductive success. Evolutionary Ecology Research 4: 759–778.
Anderson, R. B. & S. P. Lawler, 2016. Behavioral changes in tadpoles after multigenerational exposure to an invasive intraguild predator. Behavioral Ecology 27: 1790–1796.
Appleton, R. D. & R. A. Palmer, 1988. Water-borne stimuli released by predator crabs and damage prey induce more predator-resistant shells in a marine gastropod. Proceedings of the National Academy of Sciences 85: 4387–4391.
Bernot, R. J. & A. M. Turner, 2001. Predator identity and trait-mediated indirect effects in a littoral food web. Oecologia 129: 139–146.
Bookstein, F. L., 1997. Morphometric tools for landmark data: geometry and biology. Cambridge University Press, Cambridge.
Boyce, M. S., C. J. Johnson, E. H. Merrill, S. E. Nielsen, E. J. Solberg & B. Moorter, 2016. Can habitat selection predict abundance? Journal of Animal Ecology 85: 11–20.
Brown, J. S., B. P. Kotler & A. Bouskila, 2001. Ecology of fear: foraging games between predators and prey with pulsed resources. Annales Zoologici Fennici 38: 71–87.
Caldwell, G. S., 1986. Predation as a selective force on foraging herons: effects of plumage color and flocking. The Auk 103: 494–505.
Chivers, D. P. & R. J. F. Smith, 1998. Chemical alarm signalling in aquatic predator-prey systems: a review and prospectus. Ecoscience 5: 338–352.
Costello, D. M. & M. J. Michel, 2013. Predator-induced defenses in tadpoles confound body stoichiometry predictions of the general stress paradigm. Ecology 94: 2229–2236.
Crossland, M. R. & C. Azevedo-Ramos, 1999. Effects of Bufo (Anura: bufonidae) toxin on tadpoles from native and exotic Bufo habitats. Herpetologica 55: 192–199.
Davies, G. M. & A. Gray, 2015. Don’t let spurious accusations of pseudoreplication limit our ability to learn from natural experiments (and other messy kinds of ecological monitoring). Ecology and Evolution 5: 5295–5304.
DeWitt, T. J. & R. B. Langerhans, 2003. Multiple prey traits, multiple predators: keys to understanding complex community dynamics. Journal of Sea Research 49: 143–145.
DeWitt, T. J., B. W. Robinson & D. S. Wilson, 2000. Functional diversity among predators of a freshwater snail imposes an adaptive trade-off for shell morphology. Evolutionary Ecology Research 2: 129–148.
Edelaar, P., A. M. Siepielski & J. Clobert, 2008. Matching habitat choice causes directed gene flow: a neglected dimension in evolution and ecology. Evolution 62: 2462–2472.
Eklöv, P. & R. Svanbak, 2006. Predation risk influences adaptive morphological variation in fish populations. The American Naturalist 167: 440–452.
Ferguson, S. H., A. T. Bergerud & R. Ferguson, 1988. Predation risk and habitat selection in the persistence of a remnant caribou population. Oecologia 76: 236–245.
Godin, J. & M. Keenleyside, 1984. Foraging on on patchily distributed prey by a cichlid fish (Teleosteicichlidae): a test of the ideal free distribution theory. Animal Behaviour 32: 120–131.
Gosner, K. L., 1960. A simplified table for staging anuran embryos larvae with notes on identification. Herpetologica 16: 183–190.
Gotceitas, V., 1990. Foraging and predator avoidance: a test of a patch choice model with juvenile bluegill sunfish. Oecologia 83: 346–351.
Guariento, R. D., B. Luttbeg, T. Mehner & F. A. Esteves, 2014. The effect of predation pressure and predator adaptive foraging on the relative importance of consumptive and non-consumptive predator net effects in a freshwater model system. Oikos 123: 705–713.
Guariento, R. D., B. Luttbeg, L. S. Carneiro & A. Caliman, 2018. Prey adaptive behaviour under predation risk modify stoichiometry predictions of predator-induced stress paradigms. Functional Ecology 32: 1631–1643.
Guariento, R. D., L. S. Carneiro, F. A. Esteves, J. S. Jorge & A. Caliman, 2015. Conspecific density affects predator-induced prey phenotypic plasticity. Ecosphere 6: 1–12.
Gunzburger, M. S. & J. Travis, 2004. Evaluating predation pressure on green treefrog larvae across a habitat gradient. Oecologia 140: 422–429.
Hammill, E. & A. P. Beckerman, 2010. Reciprocity in predator-prey interactions: exposure to defended prey and predation risk affects intermediate predator life history and morphology. Oecologia 163: 193–202.
Hammond, J. I., B. Luttbeg & A. Sih, 2007. Predator and prey space use: dragonflies and tadpoles in an interactive game. Ecology 88: 1525–1535.
Hanski, I. & O. Ovaskainen, 2000. The metapopulation capacity of a fragmented landscape. Nature 404: 755.
Heithaus, M. R. & L. M. Dill, 2002. Food availability and tiger shark predation risk. Ecology 83: 480–491.
Hero, J. M., W. E. Magnusson, C. F. Rocha & C. P. Catterall, 2001. Antipredator defenses influence the distribution of amphibian prey species in the central Amazon rain forest. Biotropica 33: 131–141.
Hossie, T., K. Landolt & D. L. Murray, 2017. Determinants and co-expression of anti-predator responses in amphibian tadpoles: a meta-analysis. Oikos 126: 173–184.
Howe, N. R. & Y. M. Sheikh, 1975. Anthopleurine: a sea anemone alarm pheromone. Science 189: 386–388.
Jara, F. G. & M. G. Perotti, 2010. Risk of predation and behavioural response in three anuran species: influence of tadpole size and predator type. Hydrobiologia 644: 313–324.
Kacelnik, A., J. R. Krebs & C. Bernstein, 1992. The ideal free distribution and predator-prey populations. Trends in Ecology & Evolution 7: 50–55.
Kerfoot, W. C., 1987. Translocation experiments: bosmina responses to copepod predation. Ecology 68: 596–610.
Kotler, B. P. & L. Blaustein, 1995. Titrating food and safety in a heterogenous environment: when are the risky and safety patches of equal value? Oikos 74: 251–258.
Krivan, V., 1997. Dynamic ideal free distribution: effects of optimal patch choice on predator-prey dynamics. The American Naturalist 149: 164–178.
Krivan, V., 2003. Ideal free distributions when resources undergo population dynamics. Theoretical Population Biology 64: 25–38.
Lima, S. L., 1998. Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Advances in the Study of Behavior 27: 215–290.
Lima, S. L. & L. M. Dill, 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Canadian Journal of Zoology 68: 619–640.
Lima, S. L. & P. A. Bednekoff, 1999. Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. The American Naturalist 153: 649–659.
Luttbeg, B., J. I. Hammond & A. Sih, 2008. Dragonfly larvae and tadpole frog space use games in varied light conditions. Behavioral Ecology 20: 13–21.
Luttbeg, B. & A. Sih, 2004. Predator and prey habitat selection games: the effects of how prey balance foraging and predation risk. Israel Journal of Zoology 50: 233–254.
McCollum, S. A. & J. D. Leimberger, 1997. Predator-induced morphological changes in an amphibian: predation by dragonflies affects tadpole shape and color. Oecologia 109: 615–621.
McCoy, M. W., B. M. Bolker, C. W. Osenberg, B. G. Miner & J. R. Vonesh, 2006. Size correction: comparing morphological traits among populations and environments. Oecologia 148: 547–554.
McDiarmid, R. W. & R. Altig, 1999. Tadpoles: The Biology of Anuran Larvae. The University of Chicago Press, Chicago.
McIvor, C. C. & W. E. Odum, 1988. Food, predation risk, and microhabitat selection in a marsh fish assemblage. Ecology 69: 1341–1351.
Nomura, F., V. H. M. Prado, F. R. Silva, R. E. Borges, N. Y. N. Dias & D. D. C. Rossa-Feres, 2011. Are you experienced? Predator type and predator experience trade-offs in relation to tadpole mortality rates. Journal of Zoology 284: 144–150.
Pease, C. M., R. Lande & J. J. Bull, 1989. A model of population growth, dispersal and evolution in a changing environment. Ecology 70: 1657–1664.
Peckarsky, B. L., 1982. Aquatic insect predator-prey relations. BioScience 32: 261–266.
Perotti, M. G., L. A. Fitzgerald, L. Moreno & M. Pueta, 2006. Behavioral responses of Bufo arenarum tadpoles to odonate naiad predation. Herpetological Conservation and Biolology 1: 117–120.
Peterson, C. H. & G. A. Skilleter, 1994. Control of foraging behavior of individuals within an ecosystem context: the clam Macoma balthica, flow environment, and siphon-cropping fishes. Oecologia 100: 256–267.
Pigliucci, M., 2003. Phenotypic integration: studying the ecology and evolution of complex phenotypes. Ecology Letters 6: 265–272.
Pigliucci, M., 2005. Evolution of phenotypic plasticity: where are we going now? Trends in Ecology and Evolution 20: 481–486.
Prevedello, J. A., G. Forero-Medina & M. V. Vieira, 2010. Movement behaviour within and beyond perceptual ranges in three small mammals: effects of matrix type and body mass. Journal of Animal Ecology 79: 1315–1323.
R Development Core Team, 2016. R: a langauge and environment for statistical coumputing. R Foundation for Statistical Computing, Vienna, Austria. http://R-project.org.
Ravigné, V., U. Dieckmann & I. Olivieri, 2009. Live where you thrive: joint evolution of habitat choice and local adaptation facilitates specialization and promotes diversity. The American Naturalist 174: E141–E169.
Relyea, R. A., 2001. Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82: 523–540.
Relyea, R. A. & J. T. Hoverman, 2003. The impact of larval predators and competitors on the morphology and fitness of juvenile treefrogs. Oecologia 134: 596–604.
Repka, S. & K. Pihlajamaa, 1996. Predator-induced phenotypic plasticity in Daphnia pulex: uncoupling morphological defenses and life history shifts. Hydrobiologia 339: 67–71.
Rodrigues, M. E. & F. O. Roque, 2017. Checklist de Odonata do estado de Mato Grosso do Sul, Brasil. Iheringia Série Zoologia 107: e2017117.
Schmidt, K. A., J. M. Earnhardt, J. S. Brown & R. D. Holt, 2000. Habitat selection under temporal heterogeneity: exorcizing the ghost of competition past. Ecology 81: 2622–2630.
Scrimgeour, G. J., J. M. Culp & F. J. Wrona, 1994. Feeding while avoiding predators: evidence for a size-specific trade-off by a lotic mayfly. Journal of the North American Benthological Society 13: 368–378.
Seebacher, F., C. R. White & C. E. Franklin, 2015. Physiological plasticity increases resilience of ectothermic animals to climate change. Nature Climate Change 5: 61–66.
Shrader, A. M., G. I. H. Kerley, J. S. Brown & B. P. Kotler, 2012. Patch use in free-ranging goats: does a large mammalian herbivore forage like other central place foragers? Ethology 118: 967–974.
Skelly, D. K. & E. E. Werner, 1990. Behavioral and life-historical responses of larval American toads to an odonate predator. Ecology 71: 2313–2322.
Souza, F. L., C. P. A. Prado, J. L. M. M. Sugai, V. L. Ferreira, C. Aoki, P. Landgref-Filho, C. Strüssmann, R. W. Ávila, D. J. Rodrigues, N. R. Albuquerque, J. Terra, M. Uetanabaro, A. F. Béda, L. Piatti, R. A. Kawashita-Ribeiro, M. Delatorre, G. F. Faggioni, S. D. B. Demczuk & S. Duleba, 2017. Diversidade de anfíbios do Estado de Mato Grosso do Sul, Brasil. Iheringia Série Zoologia 107: e2017152.
Stav, G., B. P. Kotler & L. Blaustein, 2007. Direct and indirect effects of dragonfly (Anax imperator) nymphs on green toad (Bufo viridis) tadpoles. Hydrobiologia 579: 85–93.
Takahara, T., H. Doi, Y. Kohmatsu & R. Yamaoka, 2013. Different chemical cues originating from a shared predator induce common defense responses in two prey species. Animal Cognition 16: 147–153.
Turkia, T., E. Korpimäki, A. Villers & V. Selonen, 2018. Predation risk landscape modifies flying and red squirrel nest site occupancy independently of habitat amount. PLoS ONE 13: e0194624.
Van Baalen, M. & M. W. Sabelis, 1993. Coevolution of patch selection strategies of predator and prey and the consequences for ecological stability. The American Naturalist 142: 646–670.
Van Buskirk, J. & M. Arioli, 2002. Dosage response of an induced defence: how sensitive are tadpoles to predation risk? Ecology 83: 1580–1585.
Van Buskirk, J., P. Anderwald, S. Lüpold, L. Reinhardt & H. Schuler, 2003. The lure effect, tadpole tail shape, and the target of dragonfly strikes. Journal of Herpetology 37: 420–424.
Van Buskirk, J., A. Krügel, J. Kunz, F. Miss & A. Stamm, 2014. The rate of degradation of chemical cues indicating predation risk: an experiment and review. Ethology 120: 942–949.
Wassersug, R. J., 1971. On the comparative palatability of some dry-season tadpoles from costa Rica. American Midland Naturalist 86: 101–109.
Wells, K. D., 2010. The Ecology and Behavior of Amphibians. University of Chicago Press, Chicago.
Werner, E. E., J. F. Gilliam, D. J. Hall & G. G. Mittelbach, 1983. An experimental test of the effects of predation risk on habitat use in fish. Ecology 64: 1540–1548.
Winandy, L., P. Legrand & M. Denoël, 2017. Habitat selection and reproduction of newts in networks of fish and fishless aquatic patches. Animal Behaviour 123: 107–115.
Wong, B. B. M. & U. Candolin, 2015. Behavioral responses to changing environments. Behavioral Ecology 26: 665–673.
Acknowledgements
We are grateful to Jefferson Medina, Nathalia Mangini, and Beatriz Carneiro for their support in field work and the montage of the experiment. We thank the Use Animal Ethics Committee from Federal University of Mato Grosso do Sul by the approval of this project (protocol#732/2015). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. DJS thanks CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for his research fellowship (311492/2017-7). We also thank Dr. Luiz Gustavo de Oliveira-Santos for the helpful suggestions along the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Lee B. Kats
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10750_2019_3962_MOESM2_ESM.docx
Temporal tendency of tadpoles to occupy the high resources patches in absence of predators. Online Resource 2 (DOCX 58 kb)
Rights and permissions
About this article
Cite this article
Pacheco, E.O., Almeida-Gomes, M., Santana, D.J. et al. Space use and phenotypic plasticity in tadpoles under predation risk. Hydrobiologia 837, 77–86 (2019). https://doi.org/10.1007/s10750-019-3962-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-3962-3