Abstract
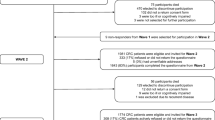
Missing responses for health-related quality of life (HRQL) outcomes are common in clinical trials and may introduce bias as such data are often not missing at random. To evaluate the missingness (dropout) effect when comparing two treatment groups in a longitudinal randomized trial, we analyzed the Functional Assessment of Cancer Therapy Trial Outcome Index (TOI) change over 12 months for newly diagnosed patients with chronic myeloid leukemia. HRQL assessment was expected at baseline and months 1, 2, 3, 4, 5, 6, 9 and 12. We defined completers as those with baseline and month 12 TOI, and dropouts as all others as long as they had a baseline score. We defined censoring time as the time interval between baseline and the scheduled month 12 visit dates and approximate time-to-dropout as the time interval from baseline to the midpoint between date of the last reported TOI and the scheduled next visit date. A mixed-effects model was first built to assess treatment effect; a pattern-mixture model and a joint model were then built to account for non-ignorable dropout. Intermittent missing data were assumed to be missing at random. A square root transformation of TOI scores was taken to fulfill the normality and homogeneity assumption at each time point in all the models. The mixed-effects model revealed significant (P < 0.001) between-group differences at each visit except for baseline. The joint model generated similar parameter estimates as the separate longitudinal and survival sub-models with a significant association parameter (P = 0.039) indicating negative association between slope of TOI and hazard of dropout and thus non-ignorable dropout. The pattern-mixture model parameter estimates were fairly similar to those generated from the joint model. When non-ignorable missing data exist in longitudinal studies, a joint model is useful to quantify the relationship between dropout and outcome. In addition, it is important to examine underlying assumptions and utilize multiple missing data models including the pattern mixture model to assess sensitivity of model based inference to assumptions about missing mechanisms.



Similar content being viewed by others
References
Bacik, J., Mazumdar, M., Murphy, B.A., Fairclough, D.L., Eremenco, S., Mariani, T., Motzer, R.J., Cella, D.: The functional assessment of cancer therapy-BRM (FACT-BRM): a new tool for the assessment of quality of life in patients treated with biologic response modifiers. Qual. Life Res. 13(1), 137–154 (2004)
Curran, D., Molenberghs, G., Thijs, H., Verbeke, G.: Sensitivity analysis for pattern mixture models. J. Biopharm. Stat. 14(1), 125–143 (2004)
Daniels, J.D., Hogan, J.W.: Missing Data in Longitudinal Studies. Chapman and Hall/CRC, London (2008)
Demirtas, H.: Multiple imputation under Bayesianly smoothed pattern-mixture models for non-ignorable drop-out. Stat. Med. 24(15), 2345–2363 (2005)
Demirtas, H., Schafer, J.L.: On the performance of random-coefficient pattern-mixture models for non-ignorable drop-out. Stat. Med. 22(16), 2553–2575 (2003)
Fairclough, D., Thijs, H., Hung, I.C., Finnern, H., Wu, A.: Handling missing quality of life data in HIV clinical trials: what is practical? Qual. Life Res. 17, 61–73 (2008)
Guo, X., Carlin, B.: Separate and joint modeling of longitudinal and event time data using standard computer packages. Am. Stat. 58, 16–24 (2004), http://www.biostat.umn.edu/~brad/software.html. Accessed 23 December 2009
Hedeker, D., Gibbons, R.D.: Longitudinal Data Analysis. Wiley, Hoboken (2006)
Hedeker, D., Gibbons, R.D.: Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol. Methods 2(1), 64–78 (1997)
Hogan, J., Laird, N.: Mixture models for the joint distribution of repeated measures and event times. Stat. Med. 16, 239–257 (1997)
Hosmer, D., Lemeshow, L.: Applied survival analysis: regression modeling of time to event data. Wiley, New York (1999)
Hsieh, F., Tseng, Y.K., Wang, J.L.: Joint modeling of survival and longitudinal data: likelihood approach revisited. Biometrics 62, 1037–1043 (2006)
Huang, X., Stefanski, L.A., Davidian, M.: Latent-model robustness in joint models for a primary endpoint and a longitudinal process. Biometrics 65, 719–727 (2009)
Ibrahim, J.G., Molenberghs, G.: Missing data methods in longitudinal studies: a review. Test 18, 1–43 (2009)
Li, H., Heitjan, D.: A pattern–mixture model for the analysis of censored quality-of-life data. Stat. Med. 25, 1533–1546 (2006)
Little, R.J.A.: Modeling the drop-out mechanism in repeated-measures studies. JASA 90, 1112–1121 (1995)
Little, R.J.A., Rubin, D.B.: Statistical Analysis with Missing Data, 2nd edn. Wiley, New York (2002)
Pauler, K., McCoy, S., Moinpour, C.: Pattern mixture models for longitudinal quality of life studies in advanced state disease. Stat. Med. 22, 795–809 (2003)
Rizopoulos, D., Verbeke, G., Molenberghs, G.: Shared parameter models under random effects misspecification. Biometrika 95, 1–12 (2008)
Schluchter, M.: Methods for the analysis of informatively censored longitudinal data. Stat Med. 11, 1861–1870 (1992)
Tierney, L., Kadane, J.B.: Accurate approximation for posterior moments and marginal densities. JASA 81, 82–86 (1986)
Tsiatis, A.A., Davidian, M.: Joint modelling of longitudinal and time-to-event data: an overview. Statistica Sinica 14, 809–834 (2004)
Vonesh, E., Greene, T., Schluchter, M.: Shared parameter models for the joint analysis of longitudinal data and event times. Stat. Med. 25(1), 143–163 (2006)
Webster, K., Cella, D., Yost, K.: The functional assessment of chronic illness therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual. Life Outcomes 1, 79 (2003)
Zeng, D., Cai, J.: Simultaneous modelling of survival and longitudinal data with an application to repeated quality of life measures. Lifetime Data Anal. 11, 151–174 (2005)
Acknowledgments
This project was funded in part by an award from the Davis Family Endowment for Outcomes Research. Data were kindly provided by Novartis. We want to thank the two referees and the editors for their constructive comments which improve the manuscript substantially.
Author information
Authors and Affiliations
Corresponding author
Additional information
Based on presentation at the 8th International Conference on Health Policy Statistics, Washington, DC, U.S.A., 20th–22nd January 2010.
Rights and permissions
About this article
Cite this article
Du, H., Hahn, E.A. & Cella, D. The impact of missing data on estimation of health-related quality of life outcomes: an analysis of a randomized longitudinal clinical trial. Health Serv Outcomes Res Method 11, 134–144 (2011). https://doi.org/10.1007/s10742-011-0074-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10742-011-0074-6