Abstract
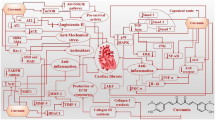
Cardiac fibrosis stems from the changes in the expression of fibrotic genes in cardiac fibroblasts (CFs) in response to the tissue damage induced by various cardiovascular diseases (CVDs) leading to their transformation into active myofibroblasts, which produce high amounts of extracellular matrix (ECM) proteins leading, in turn, to excessive deposition of ECM in cardiac tissue. The excessive accumulation of ECM elements causes heart stiffness, tissue scarring, electrical conduction disruption and finally cardiac dysfunction and heart failure. Curcumin (Cur; also known as diferuloylmethane) is a polyphenol compound extracted from rhizomes of Curcuma longa with an influence on an extensive spectrum of biological phenomena including cell proliferation, differentiation, inflammation, pathogenesis, chemoprevention, apoptosis, angiogenesis and cardiac pathological changes. Cumulative evidence has suggested a beneficial role for Cur in improving disrupted cardiac function developed by cardiac fibrosis by establishing a balance between degradation and synthesis of ECM components. There are various molecular mechanisms contributing to the development of cardiac fibrosis. We presented a review of Cur effects on cardiac fibrosis and the discovered underlying mechanisms by them Cur interact to establish its cardio-protective effects.


Similar content being viewed by others
References
Grimaldi V, De Pascale MR, Zullo A, Soricelli A, Infante T, Mancini FP, Napoli C (2017) Evidence of epigenetic tags in cardiac fibrosis. J Cardiol 69(2):401–408
Russo I, Frangogiannis NG (2016) Diabetes-associated cardiac fibrosis: cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol 90:84–93. https://doi.org/10.1016/j.yjmcc.2015.12.011
Tao H, Shi K-H, Yang J-J, Huang C, Liu L-P, Li J (2013) Epigenetic regulation of cardiac fibrosis. Cell Signal 25(9):1932–1938
Wang NP, Wang ZF, Tootle S, Philip T, Zhao ZQ (2012) Curcumin promotes cardiac repair and ameliorates cardiac dysfunction following myocardial infarction. Br J Pharmacol 167(7):1550–1562
van Putten S, Shafieyan Y, Hinz B (2016) Mechanical control of cardiac myofibroblasts. J Mol Cell Cardiol 93:133–142. https://doi.org/10.1016/j.yjmcc.2015.11.025
Creemers EE, van Rooij E (2016) Function and therapeutic potential of noncoding RNAs in cardiac fibrosis. Circ Res 118(1):108–118
Micheletti R, Plaisance I, Abraham BJ, Sarre A, Ting C-C, Alexanian M, Maric D, Maison D, Nemir M, Young RA (2017) The long noncoding RNA Wisper controls cardiac fibrosis and remodeling. Sci Transl Med 9(395):eaai9118
Xiao J, Sheng X, Zhang X, Guo M, Ji X (2016) Curcumin protects against myocardial infarction-induced cardiac fibrosis via SIRT1 activation in vivo and in vitro. Drug design, development and therapy 10:1267
Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S, Aggarwal BB (2017) Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol 174(11):1325–1348
Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendía LE, Sahebkar A (2017) Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res 67(4):244–251. https://doi.org/10.1055/s-0043-100019
Rezaee R, Momtazi AA, Monemi A, Sahebkar A (2017) Curcumin: A potentially powerful tool to reverse cisplatin-induced toxicity. Pharmacol Res 117:218–227. https://doi.org/10.1016/j.phrs.2016.12.037
Karimian MS, Pirro M, Majeed M, Sahebkar A (2017) Curcumin as a natural regulator of monocyte chemoattractant protein-1. Cytokine Growth F R 33:55–63. https://doi.org/10.1016/j.cytogfr.2016.10.001
Sahebkar A, Cicero AFG, Simental-Mendia LE, Aggarwal BB, Gupta SC (2016) Curcumin downregulates human tumor necrosis factor-alpha levels: a systematic review and meta-analysis ofrandomized controlled trials. Pharmacol Res 107:234–242. https://doi.org/10.1016/j.phrs.2016.03.026
Wongcharoen W, Phrommintikul A (2009) The protective role of curcumin in cardiovascular diseases. Int J Cardiol 133(2):145–151
Fang G, Chen S, Huang Q, Chen L, Liao D (2018) Curcumin suppresses cardiac fibroblasts activities by regulating the proliferation and cell cycle via the inhibition of the p38 MAPK/ERK signaling pathway. Mol Med Rep 18(2):1433–1438
Meng Z, Yu X-h, Chen J, Li L, Li S (2014) Curcumin attenuates cardiac fibrosis in spontaneously hypertensive rats through PPAR-γ activation. Acta Pharmacol Sin 35(10):1247
Leask A (2010) Potential therapeutic targets for cardiac fibrosis: TGFβ, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res 106(11):1675–1680
Valente AJ, Sakamuri SS, Siddesha JM, Yoshida T, Gardner JD, Prabhu R, Siebenlist U, Chandrasekar B (2013) TRAF3IP2 mediates interleukin-18-induced cardiac fibroblast migration and differentiation. Cell Signal 25(11):2176–2184
Zhao T, Zhao W, Chen Y, Li VS, Meng W, Sun Y (2013) Platelet-derived growth factor-D promotes fibrogenesis of cardiac fibroblasts. Am J Phys Heart Circ Phys 304(12):H1719–H1726
Shimosawa T (2013) Salt, the renin–angiotensin–aldosterone system and resistant hypertension. Hypertens Res 36(8):657
de Cavanagh EM, Ferder M, Inserra F, Ferder L (2009) Angiotensin II, mitochondria, cytoskeletal, and extracellular matrix connections: an integrating viewpoint. Am J Phys Heart Circ Phys 296(3):H550–H558
Siddesha JM, Valente AJ, Sakamuri SS, Yoshida T, Gardner JD, Somanna N, Takahashi C, Noda M, Chandrasekar B (2013) Angiotensin II stimulates cardiac fibroblast migration via the differential regulation of matrixins and RECK. J Mol Cell Cardiol 65:9–18. https://doi.org/10.1016/j.yjmcc.2013.09.015
Crowley MJ, Powers BJ, Myers ER, McBroom AJ, Sanders GD (2012) Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treatment of ischemic heart disease: future research needs prioritization. Am Heart J 163(5):777–782 e778
Ghosh AK, Vaughan DE (2012) PAI-1 in tissue fibrosis. J Cell Physiol 227(2):493–507
Weisberg AD, Albornoz F, Griffin JP, Crandall DL, Elokdah H, Fogo AB, Vaughan DE, Brown NJ (2005) Pharmacological inhibition and genetic deficiency of plasminogen activator inhibitor-1 attenuates angiotensin II/salt-induced aortic remodeling. Arterioscler Thromb Vasc Biol 25(2):365–371
Khan R, Sheppard R (2006) Fibrosis in heart disease: understanding the role of transforming growth factor-β1 in cardiomyopathy, valvular disease and arrhythmia. Immunology 118(1):10–24
Hein S, Arnon E, Kostin S, Schönburg M, Elsässer A, Polyakova V, Bauer EP, Klövekorn W-P, Schaper J (2003) Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation 107(7):984–991
Ghosh AK, Varga J (2007) The transcriptional coactivator and acetyltransferase p300 in fibroblast biology and fibrosis. J Cell Physiol 213(3):663–671
Daniels A, Van Bilsen M, Goldschmeding R, Van Der Vusse G, Van Nieuwenhoven F (2009) Connective tissue growth factor and cardiac fibrosis. Acta Physiol 195(3):321–338
Gao D-F, Niu X-L, Hao G-H, Peng N, Wei J, Ning N, Wang N-P (2007) Rosiglitazone inhibits angiotensin II-induced CTGF expression in vascular smooth muscle cells––role of PPAR-γ in vascular fibrosis. Biochem Pharmacol 73(2):185–197
Derosa G, Maffioli P (2012) Peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists on glycemic control, lipid profile and cardiovascular risk. Curr Mol Pharmacol 5(2):272–281
Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM (2013) PPARγ signaling and metabolism: the good, the bad and the future. Nat Med 19(5):557
Wei W-Y, Zhang N, Li L-L, Ma Z-G, Xu M, Yuan Y-P, Deng W, Tang Q-Z (2018) Pioglitazone alleviates cardiac fibrosis and inhibits endothelial to mesenchymal transition induced by pressure overload. Cell Physiol Biochem 45(1):26–36
Singh RM, Cummings E, Pantos C, Singh J (2017) Protein kinase C and cardiac dysfunction: a review. Heart Fail Rev 22(6):843–859
Huang K-P (1989) The mechanism of protein kinase C activation. Trends Neurosci 12(11):425–432
Singh RK, Kumar S, Gautam PK, Tomar MS, Verma PK, Singh SP, Acharya A (2017) Protein kinase C-α and the regulation of diverse cell responses. Biomolecular concepts 8(3–4):143–153
Roberts AC, Porter KE (2013) Cellular and molecular mechanisms of endothelial dysfunction in diabetes. Diabetes and Vascular Disease Research 10(6):472–482
Davis J, Molkentin JD (2014) Myofibroblasts: trust your heart and let fate decide. J Mol Cell Cardiol 70:9–18
Cicero AFG, Colletti A, Bajraktari G, Descamps O, Djuric DM, Ezhov M, Fras Z, Katsiki N, Langlois M, Latkovskis G, Panagiotakos DB, Paragh G, Mikhailidis DP, Mitchenko O, Paulweber B, Pella D, Pitsavos C, Reiner Z, Ray KK, Rizzo M, Sahebkar A, Serban MC, Sperling LS, Toth PP, Vinereanu D, Vrablík M, Wong ND, Banach M (2017) Lipid-lowering nutraceuticals in clinical practice: position paper from an international lipid expert panel. Nutr Rev 75(9):731–767. https://doi.org/10.1093/nutrit/nux047
Panahi Y, Khalili N, Sahebi E, Namazi S, Karimian MS, Majeed M, Sahebkar A (2017) Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: a randomized controlled trial. Inflammopharmacology 25(1):25–31. https://doi.org/10.1007/s10787-016-0301-4
Sahebkar A, Cicero AFG, Simental-Mendía LE, Aggarwal BB, Gupta SC (2016) Curcumin downregulates human tumor necrosis factor-α levels: a systematic review and meta-analysis ofrandomized controlled trials. Pharmacol Res 107:234–242. https://doi.org/10.1016/j.phrs.2016.03.026
Liu W, Zhai Y, Heng X, Che FY, Chen W, Sun D, Zhai G (2016) Oral bioavailability of curcumin: problems and advancements. J Drug Target 24(8):694–702
Hagl S, Kocher A, Schiborr C, Kolesova N, Frank J, Eckert GP (2015) Curcumin micelles improve mitochondrial function in neuronal PC12 cells and brains of NMRI mice - impact on bioavailability. Neurochem Int 89:234–242. https://doi.org/10.1016/j.neuint.2015.07.026
Sandhir R, Yadav A, Mehrotra A, Sunkaria A, Singh A, Sharma S (2014) Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington's disease. NeuroMolecular Med 16(1):106–118. https://doi.org/10.1007/s12017-013-8261-y
Chojnacki JE, Liu K, Yan X, Toldo S, Selden T, Estrada M, Rodríguez-Franco MI, Halquist MS, Ye D, Zhang S (2014) Discovery of 5-(4-hydroxyphenyl)-3-oxo-pentanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide as a neuroprotectant for Alzheimer's disease by hybridization of curcumin and melatonin. ACS Chem Neurosci 5(8):690–699. https://doi.org/10.1021/cn500081s
Chang CZ, Wu SC, Lin CL, Kwan AL (2015) Curcumin, encapsulated in nano-sized PLGA, down-regulates nuclear factor kappaB (p65) and subarachnoid hemorrhage induced early brain injury in a rat model. Brain Res 1608:215–224. https://doi.org/10.1016/j.brainres.2015.02.039
Jayaraj RL, Tamilselvam K, Manivasagam T, Elangovan N (2013) Neuroprotective effect of CNB-001, a novel pyrazole derivative of curcumin on biochemical and apoptotic markers against rotenone-induced SK-N-SH cellular model of Parkinson's disease. Journal of molecular neuroscience : MN 51(3):863–870. https://doi.org/10.1007/s12031-013-0075-8
Mythri RB, Harish G, Dubey SK, Misra K, Bharath MM (2011) Glutamoyl diester of the dietary polyphenol curcumin offers improved protection against peroxynitrite-mediated nitrosative stress and damage of brain mitochondria in vitro: implications for Parkinson's disease. Mol Cell Biochem 347(1–2):135–143. https://doi.org/10.1007/s11010-010-0621-4
Pan Y, Wang Y, Zhao Y, Peng K, Li W, Wang Y, Zhang J, Zhou S, Liu Q, Li X (2014) Inhibition of JNK phosphorylation by a novel curcumin analog prevents high glucose–induced inflammation and apoptosis in cardiomyocytes and the development of diabetic cardiomyopathy. Diabetes 63(10):3497–3511
Chen HJ, Yang X, Lu KQ, Lu C, Zhao YJ, Zheng SQ, Li JL, Huang ZJ, Huang Y, Zhang YL, Liang G (2017) Inhibition of high glucose-induced inflammation and fibrosis by a novel curcumin derivative prevents renal and heart injury in diabetic mice. Toxicol Lett 278:48–58. https://doi.org/10.1016/j.toxlet.2017.07.212
Li KF, Zhai MG, Jiang LQ, Song F, Zhang B, Li J, Li H, Li BY, Xia L, Xu L, Cao Y, He MS, Zhu HZ, Zhang LY, Liang HL, Jin ZX, Duan WX, Wang SW (2019) Tetrahydrocurcumin ameliorates diabetic cardiomyopathy by attenuating high glucose-induced oxidative stress and fibrosis via activating the SIRT1 pathway. Oxidative Med Cell Longev 2019:Artn 6746907. https://doi.org/10.1155/2019/6746907
Tang Y, Bao M, Yang B, Zhang Y, Zhang B, Zhou Q, Chen J, Huang C (2009) Curcumin attenuates left ventricular dysfunction and remodeling in rabbits with chronic heart failure. Zhonghua xin xue guan bing za zhi 37(3):262–267
Nakayama N, Nakamura T, Okada H, Iwaki S, Sobel BE, Fujii S (2011) Modulators of induction of plasminogen activator inhibitor type-1 in HepG2 cells by transforming growth factor-β. Coron Artery Dis 22(7):468–478
Liu H, Liu A, Shi C, Li B (2016) Curcumin suppresses transforming growth factor-β1-induced cardiac fibroblast differentiation via inhibition of Smad-2 and p38 MAPK signaling pathways. Experimental and therapeutic medicine 11(3):998–1004
Zeng C, Zhong P, Zhao Y, Kanchana K, Zhang Y, Khan ZA, Chakrabarti S, Wu L, Wang J, Liang G (2015) Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-κB both in vitro and in vivo. J Mol Cell Cardiol 79:1–12
Soetikno V, Sari FR, Sukumaran V, Lakshmanan AP, Mito S, Harima M, Thandavarayan RA, Suzuki K, Nagata M, Takagi R (2012) Curcumin prevents diabetic cardiomyopathy in streptozotocin-induced diabetic rats: possible involvement of PKC–MAPK signaling pathway. Eur J Pharm Sci 47(3):604–614
Guo S, Meng XW, Yang XS, Liu XF, Ou-Yang CH, Liu C (2018) Curcumin administration suppresses collagen synthesis in the hearts of rats with experimental diabetes. Acta Pharmacol Sin 39(2):195–204. https://doi.org/10.1038/aps.2017.92
Bugyei-Twum A, Advani A, Advani SL, Zhang Y, Thai K, Kelly DJ, Connelly KA (2014) High glucose induces Smad activation via the transcriptional coregulator p300 and contributes to cardiac fibrosis and hypertrophy. Cardiovasc Diabetol 13(1):89. https://doi.org/10.1186/1475-2840-13-89
Chung CC, Kao YH, Liou LP, Chen YJ (2014) Curcumin suppress cardiac fibroblasts activities by regulating proliferation, migration, and the extracellular matrix. Acta Cardiologica Sinica 30(5):474–482
Kim YS, Kwon JS, Cho YK, Jeong MH, Cho JG, Park JC, Kang JC, Ahn Y (2012) Curcumin reduces the cardiac ischemia–reperfusion injury: involvement of the toll-like receptor 2 in cardiomyocytes. J Nutr Biochem 23(11):1514–1523
Yu W, Wu J, Cai F, Xiang J, Zha W, Fan D, Guo S, Ming Z, Liu C (2012) Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS One 7(12):e52013. https://doi.org/10.1371/journal.pone.0052013
Sunagawa Y, Sono S, Katanasaka Y, Funamoto M, Hirano S, Miyazaki Y, Hojo Y, Suzuki H, Morimoto E, Marui A (2014) Optimal dose-setting study of curcumin for improvement of left ventricular systolic function after myocardial infarction in rats. J Pharmacol Sci 126(4):329–336
Pang X-F, Zhang L-H, Bai F, Wang N-P, Garner RE, McKallip RJ, Zhao Z-Q (2015) Attenuation of myocardial fibrosis with curcumin is mediated by modulating expression of angiotensin II AT1/AT2 receptors and ACE2 in rats. Drug design, development and therapy 9:6043
Allen S, Liu YG, Scott E (2016) Engineering nanomaterials to address cell-mediated inflammation in atherosclerosis. Regen Eng Transl Med 2(1):37–50. https://doi.org/10.1007/s40883-016-0012-9
Liu R, Zhang H, Yang J, Wang J, Liu J, Li C (2018) Curcumin alleviates isoproterenol-induced cardiac hypertrophy and fibrosis through inhibition of autophagy and activation of mTOR. Eur Rev Med Pharmacol Sci 22(21):7500–7508
Ozawa H, Imaizumi A, Sumi Y, Hashimoto T, Kanai M, Makino Y, Tsuda T, Takahashi N, Kakeya H (2017) Curcumin β-D-glucuronide plays an important role to keep high levels of free-form curcumin in the blood. Biol Pharm Bull 40(9):1515–1524
Schiborr C, Eckert GP, Rimbach G, Frank J (2010) A validated method for the quantification of curcumin in plasma and brain tissue by fast narrow-bore high-performance liquid chromatography with fluorescence detection. Anal Bioanal Chem 397(5):1917–1925
Feng T, Wei Y, Lee RJ, Zhao L (2017) Liposomal curcumin and its application in cancer. Int J Nanomedicine 12:6027–6044. https://doi.org/10.2147/IJN.S132434
Tsuda T (2018) Curcumin as a functional food-derived factor: degradation products, metabolites, bioactivity, and future perspectives. Food Funct 9(2):705–714
Mirzaei H, Shakeri A, Rashidi B, Jalili A, Banikazemi Z, Sahebkar A (2017) Phytosomal curcumin: a review of pharmacokinetic, experimental and clinical studies. Biomed Pharmacother 85:102–112. https://doi.org/10.1016/j.biopha.2016.11.098
Wang Y, Zhou S, Sun W, McClung K, Pan Y, Liang G, Tan Y, Zhao Y, Liu Q, Sun J (2014) Inhibition of JNK by novel curcumin analog C66 prevents diabetic cardiomyopathy with a preservation of cardiac metallothionein expression. American Journal of Physiology-Endocrinology and Metabolism 306(11):E1239–E1247
Li C, Miao X, Lou Y, Lu Z, Adhikari BK, Wang Y, Liu Q, Sun J, Wang Y (2018) Cardioprotective effects of the novel curcumin analogue C66 in diabetic mice is dependent on JNK 2 inactivation. J Cell Mol Med 22(12):6314–6326
Bugyei-Twum A, Abadeh A, Thai K, Zhang Y, Mitchell M, Kabir G, Connelly KA (2016) Suppression of NLRP3 Inflammasome activation ameliorates chronic kidney disease-induced cardiac fibrosis and diastolic dysfunction. Sci Rep 6:39551. https://doi.org/10.1038/srep39551
Sunagawa Y, Morimoto T, Wada H, Takaya T, Katanasaka Y, Kawamura T, Yanagi S, Marui A, Sakata R, Shimatsu A, Kimura T, Kakeya H, Fujita M, Hasegawa K (2011) A natural p300-specific histone acetyltransferase inhibitor, curcumin, in addition to angiotensin-converting enzyme inhibitor, exerts beneficial effects on left ventricular systolic function after myocardial infarction in rats. Circ J 75(9):2151–2159. https://doi.org/10.1253/circj.CJ-10-1072
Ma J, Ma S-y, Ding C-h (2017) Curcumin reduces cardiac fibrosis by inhibiting myofibroblast differentiation and decreasing transforming growth factor β1 and matrix metalloproteinase 9/tissue inhibitor of metalloproteinase 1. Chin J Integr Med 23(5):362–369
Funding
Financial support was from the National Institute for Medical Research Development (NIMAD), Tehran, Iran (Grant no: 943771).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethics approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gorabi, A.M., Hajighasemi, S., Kiaie, N. et al. Anti-fibrotic effects of curcumin and some of its analogues in the heart. Heart Fail Rev 25, 731–743 (2020). https://doi.org/10.1007/s10741-019-09854-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-019-09854-6