Abstract
Infectious myocarditis is the result of an immune response to a microbial infection of the heart. The blood vessels of the heart, both the intramyocardial microvasculature and the large epicardial coronary arteries, play an important role in the pathogenesis of infectious myocarditis. First of all, in addition to cardiomyocytes, endothelial cells of the cardiac (micro)vasculature are direct targets for infection. Moreover, through the expression of adhesion molecules and antigen presenting Major Histocompatibility Complex molecules, the blood vessels assist in shaping the cellular immune response in infectious myocarditis. In addition, damage and dysfunction of the cardiac (micro)vasculature are associated with thrombus formation as well as aberrant regulation of vascular tone including coronary vasospasm. These in turn can cause cardiac perfusion abnormalities and even myocardial infarction. In this review, we will discuss the role of the cardiac (micro)vasculature in the pathogenesis of infectious myocarditis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
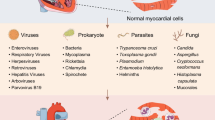
Myocarditis is an inflammatory disease of the heart that is characterized by a large diversity in symptoms varying from a symptomless course to shortness of breath and mild flu-like symptoms, chest pain, specific or a specific ECG changes, to acute heart failure and chronically to dilated cardiomyopathy. [1] In the heart, myocarditis can induce cell loss, interstitial and replacement fibrosis, wall motion abnormalities, decreased ejection fraction, and arrhythmias. [2] Moreover, myocarditis is one of the leading causes of sudden cardiac death in young adults. [3] The cause of myocarditis can among others be an allergic or toxic reaction to medicines and toxic drugs as well as autoimmune organ-specific myocarditis and systemic autoimmune diseases-associated myocarditis. However, most often, the cause of myocarditis is an infection, including viruses, bacteria, protozoa, and fungi. In the Western world, viral infection is the most common cause of myocarditis. [4] At present, over 20 different viruses have been associated with viral myocarditis. [4] In South and Central America, infection of the protozoa Trypanosoma cruzi (T. cruzi) that leads to the so-called Chagas disease is the most prominent cause of myocarditis. [4, 5] Therefore, in this review, we will focus on viral- and T. cruzi-induced myocarditis. Of note, lymphocytic myocarditis refers to cases of myocarditis with the (immuno)histological appearance of intramyocardial foci of mainly infiltrated lymphocytes, that is generally considered to be most likely of a viral cause, but were no molecular detection was preformed to prove viral presence.
Pathogenesis of viral-induced myocarditis
Most of our knowledge on the pathogenesis of viral myocarditis is obtained from studies in mice with experimental viral myocarditis. As such, we know that the pathophysiological course of viral myocarditis can be subdivided in three subsequent phases: an acute, subacute, and chronic phase. [6, 7] During the acute phase in the first days after infection, viral replication occurs within organs, including the heart. [6] In the subacute phase, approximately a week after infection, an immune response is activated in the heart aimed at clearing the virus resulting in increased levels of cytokines and immune cell infiltration into the myocardium. [8] In the chronic phase, 2 weeks after infection, the virus is usually cleared, myocardial inflammation resolved, and remodeling of the myocardium occurs. The damage of the heart in viral myocarditis can be the result of direct virus-related cardiac damage, i.e., the virus infects cardiac cells and kills them, and of autoimmunity, i.e., through mechanisms such as molecular mimicry the activated immune system also attacks the heart. [8] It has to be noticed that in part of the patients with viral myocarditis inflammation in the heart does not subside, but becomes chronic. This may be related to persistent viral infection in the heart or to an ongoing autoimmune response and can lead to the development of dilated cardiomyopathy.
Pathogenesis of T. cruzi-induced myocarditis
The pathophysiological course of Chagas disease can be divided into an acute and chronic phase. The acute phase occurs 4–8 weeks after infection wherein T. cruzi spreads systemically into the heart and other organs. [5, 9] In the heart inflammation, necrosis of cardiomyocytes and amastigote nests of T. cruzi can be observed. [5] In case the parasite is not eliminated, the chronic phase of Chagas disease will develop in part of the patients. The chronic phase develops months, but usually decades after the primary infection. [10] The heart then is most commonly affected resulting in ventricular enlargement, thinning of the walls, interstitial fibrosis, mural thrombi, and arrhythmias. [11]
The blood vessels of the heart, both the intramyocardial microvasculature as well as the epicardial coronary arteries, play an important role in the pathogenesis of infectious myocarditis. They provide a barrier function to prevent blood-borne pathogens of entering the heart, but at the same time may form a prime target of infection. Moreover, they are a vital part of the post-infection immune responses in the heart. Furthermore, part of the clinical symptoms of patients with viral myocarditis or Chagas disease point to damage or dysfunction of the intramyocardial and/or epicardial blood vessels, such as coagulopathy, perfusion defects, and coronary spasms. In this review, we will discuss the structural and functional changes of the cardiac vasculature that occur with infectious myocarditis, caused by viral or T. cruzi infection, in more detail.
Effects on the endothelium
Infection of the cardiac (micro)vasculature
In infectious myocarditis, microbes such as cardiotropic viruses and T. cruzi infect the heart. However, of many myocarditis-associated viruses, detailed knowledge on which cell types are specifically infected in human infectious myocarditis remains scarce. In patients, viruses are usually identified via the detection of viral genome using polymerase chain reaction, which does not reveal the identity of the specific cells infected. [4] The loss of cardiomyocytes that occurs in infectious myocarditis suggests that the responsible microbes do infect cardiomyocytes. However, especially autopsy studies point out that there is a large variety in the amount of cardiomyocyte death in infectious myocarditis, which may be very limited, or even totally absent in case of borderline myocarditis. In addition, since autoimmunity can be triggered in infectious myocarditis, (part of the) damage to cardiomyocytes may also be the result of autoimmune responses. [12]
Another target of infection is the cardiac vasculature. To infect the heart, the endothelium of the cardiac microvasculature is the first barrier blood-borne infectious agents encounter. Of T. cruzi and more than 20 viruses that are associated with myocarditis, it is known that they can infect human and/or animal endothelial cells (Supplementary Table 1). In human autopsy material with proven myocarditis, it was demonstrated that Coxsackievirus, Cytomegalovirus, Dengue virus, Hanta virus, Herpes simplex virus, Parvovirus B19, and T. cruzi can infect the microvascular endothelial cells of the heart. [13,14,15,16,17,18,19] Also in animals, models with myocarditis, encephalomyocarditis virus, herpes simplex virus, and T. cruzi have been observed to infect cardiac endothelial cells. [20,21,22,23] Additionally, other cardiotropic viruses that are associated with myocarditis can infect cardiac endothelial cells in vivo and/or in vitro, of which is unknown whether they can infect the cardiac endothelial cells simultaneously with myocarditis. Lastly, there is an abundant number of cardiotropic viruses that are able to infect non-cardiac endothelial cells in vivo and/or in vitro that may potentially also infect cardiac endothelial cells (Supplementary Table 1).
The infection of the cardiac endothelium can cause among others endothelial activation, damage, and permeability. For instance, infection of cardiac endothelial cells in patients with viral myocarditis has shown to induce endothelial microparticles reflecting endothelial damage. [24] However, changes to the cardiac (micro)vasculature observed in infectious myocarditis may also be caused by non-viral mechanisms and that the exact cause of such changes, when encountered in vivo, is not known.
Expression of adhesion molecules on the cardiac (micro)vascular endothelium
Infection of the heart and the subsequent death of cardiac cells induce an inflammatory response via the release of cytokines and chemokines that result in the expression of adhesion molecules at the site of infection and injury (schematically represented in Fig. 1a). It has been shown in endomyocardial biopsies (EMB) of patients with lymphocytic myocarditis and of patients with chronic Chagas disease that the expressions of Intercellular Adhesion Molecule 1 (ICAM-1), Vascular Cell Adhesion Molecule 1 (VCAM-1), and E-selectin on the capillary endothelium are elevated. [25,26,27] In the EMB of patients with lymphocytic myocarditis, respectively 81 and 46% of the cardiac blood vessels were positive for ICAM-1 or VCAM-1, whereas in control tissue obtained from patients with tetralogy of Fallot, 24% of the cardiac blood vessels was positive for ICAM-1 and 10% for VCAM-1. [25] Moreover, VCAM-1 was predominantly expressed on the endothelial cells of venules at the inflammatory sites in the EMB of part of the chronic Chagas patients. Similar, also mice with T. cruzi infection express significantly more VCAM-1 and ICAM-1 on their cardiac endothelium compared to mice without infection. [28]
This increase in endothelial adhesion molecule expression in the myocardium of patients with infectious myocarditis probably is related to pro-inflammatory cytokines, as elevated levels of the pro-inflammatory cytokines tumor necrosis factor-α (TNFα) and interleukin-1 (IL-1β) were shown to increase the expression of adhesion molecules ICAM-1, VCAM-1, and E-selectin on the endothelial cells of the human coronary artery and cardiac microvasculature. [29] Indeed, in part of the patients with viral-proven myocarditis or Chagas disease, elevated levels of pro-inflammatory cytokines have been measured. [30] Moreover, at autopsy, we observed accumulation of the advanced glycation end product (AGE) Nε-(carboxyethyl)lysine (CML) on the endothelium of intramyocardial blood vessels in patients with lymphocytic myocarditis. [27] It is known that AGEs such as CML can induce the expression of adhesion molecules on endothelial cells via binding to scavenger receptors such as the receptor for AGEs (RAGE) [31], and that RAGE expression is increased in the hearts of mice after Coxsackievirus B3-induced acute myocarditis [32].
On the one hand, the presence of pathogens themselves can affect endothelial adhesion molecule expression. Indeed, in vitro studies have shown upregulation of ICAM-1 and VCAM-1 expression in human endothelial cells upon infection with myocarditis-associated viruses (adenovirus, arenavirus, coxsackievirus B3 (CVB3), cytomegalovirus, and West Nile virus) or upon infection with T. cruzi. [33, 34] Interestingly, many viruses including rhinovirus, coxsackieviruses, picorna virus, and encephalomyocarditis virus utilize adhesion molecules such as ICAM-1 and VCAM-1 as cell recognition and cell entry receptors. [35, 36] Thus, the virus-induced adhesion molecule upregulation may provide a positive feedback and exacerbate infection.
On the other hand, adhesion molecules on cardiac microvascular endothelial cells control leukocyte traffic between the blood and the myocardium. Therefore, elevated expression levels of adhesion molecules also increases the infiltration rate of inflammatory cells into the affected myocardium in patients with myocarditis, thereby facilitating the clearance of virus and virus-infected cells. However, chronically increased endothelial adhesion molecule expression in the cardiac microvasculature, whether or not through persistent viral infection [33], may facilitate autoimmune responses in the heart and the development of chronic heart failure.
Expression of major histocompatibility complex molecules on the cardiac (micro)vascular endothelium
Another set of cell surface proteins that are important in shaping the immune response in the heart in infectious myocarditis are the major histocompatibility complex (MHC) molecules or their human variant the Human Leucocyte Antigen (HLA). MHC molecules display processed self and non-self-antigens on the cell surface for the specific recognition by T cells for appropriate action in case of infection-related non-self-antigen. MHC class I molecules are expressed on all nucleated cells, including cardiac endothelial cells, whereas the expression of MHC class II molecules is usually restricted to professional antigen presenting cells.
In EMB of patients with lymphocytic or viral myocarditis and of patients with chronic Chagas disease, increased expression of MHC class I but also MHC class II molecules was observed on the endothelium of the cardiac microvasculature (Fig. 1a). [37,38,39,40] It seems likely that the cardiac microvascular endothelial cells use these MHC molecules to display antigens of the infectious agent to effector or helper T cells to control the infection. MHC class I interacts with CD8+ cytotoxic T cells that can mediate destruction of the infected cells, whereas MHC class II interacts with CD4+ helper T cells initiating a specific immunity. In addition, the control of several cardiotropic viruses, including parvovirus B19 [41], influenza [42], dengue [43], hepatitis B virus [44], and herpesviruses [45] may depend significantly on MHC class II-activated cytotoxic CD4+ effectors.
The increased expression of endothelial MHC molecules can be induced by interferon gamma (IFN-γ), a cytokine that is released commonly during virus infections. [46] Indeed, INFγ levels were shown to be increased in both mice viral and T. cruzi-induced myocarditis. [47, 48] Alternatively, the induction of MHC molecules might be directly virus related as both MHC class I and II expression was shown to be induced in endothelial cells in vitro by West Nile virus infection. [34]
The expression of MHC class II on the cardiac endothelium may also relate to the development of autoimmunity and chronic myocarditis after infectious myocarditis [49], although the precise mechanisms are not yet clear. On the one hand it was shown in mice that absence of non-hematopoietic MHC class II expression prevented the development of experimental autoimmune myocarditis [49], suggesting that the presence of MHC class II on cardiac cells elicits autoimmunity development. Moreover, the susceptibility to develop autoimmunity may be related to certain HLA alleles as the introduction of human HLA-DQ8 alleles in non-obese diabetic (NOD) mice induced spontaneous autoimmune myocarditis and dilated cardiomyopathy [50], which underscores the possibility of a genetic MHC class II-related predisposition in patients who develop chronic myocarditis and dilated cardiomyopathy after infectious myocarditis. Conversely, mice lacking MHC class II were more susceptible to long-term cardiac injury and viral persistence after Coxsackievirus B3 infection, suggesting that MHC class II-mediated immune responses are necessary to prevent chronic myocarditis. [51]
Increased permeability of the cardiac (micro)vasculature
In patients with myocarditis, an increase in intramyocardial edema is a frequently observed phenomenon during tissue characterization by cardiac magnetic resonance (CMR). [1, 52] This increase in edema points to an increased permeability of the cardiac microvasculature in patients with infectious myocarditis. In addition, increased permeability has been observed in the epicardial coronary arteries of patients with lymphocytic myocarditis. [53]
Multiple causes may underlie this increase in vascular permeability in infectious myocarditis (Fig. 1a). An increase in vascular permeability normally accompanies inflammatory responses in the underlying tissue. For instance, the elevated levels of pro-inflammatory cytokines that are present in the inflamed heart can affect the permeability of the (micro)vasculature. Also, the sometimes extensive recruitment and extravasation of immune cells into the myocardium may increase the cardiac (micro)vascular permeability. [54] Moreover, accumulation of the aforementioned AGE CML in the cardiac microvasculature may associate with increased permeability as was shown for diabetic vasculopathy hamsters. [55]
Alternatively, infection of endothelial cells, that can occur in infectious myocarditis (Supplementary Table 1), has been shown to affect vascular permeability directly. Its barrier function might decrease through infection-induced death of endothelial cells. For instance, infection of cardiac microvascular endothelial cells with Coxsackievirus B3 resulted in an increase of apoptosis-related caspase-3 activity and Bax/Bcl-2 protein ratio, coinciding with an elevated microvascular permeability. [56] Moreover, viral infection in endothelial cells was shown to induce decreased levels of VE-cadherin [57], enlarged intercellular gap junctions [56], and disruption of the tight junctions [58]. Conversely though, dengue virus infection of human endothelial cells in vitro led to an improved barrier function [59], suggesting that the mechanisms that underlie infection-induced (micro)vascular permeability may differ between infectious agents. In theory, the increased vascular permeability and loss of barrier function would facilitate the infection of the underlying tissue, as was for instance shown to occur in mice infected with T. cruzi. [60]
Proliferation of the (micro)vascular cells
Infectious myocarditis may also induce structural changes of the cardiac vasculature. In rats with T. cruzi-induced acute Chagas disease, an increase of both volume and length of blood vessels was shown within the ventricles and atria of the heart. [61] Little is known about the structural cardiac vascular changes in patients, although in patients with chronic Chagas disease increased arteriole and capillary diameters were observed within the ventricles. [62] A possible explanation for these alterations of the cardiac microvasculature is that infectious myocarditis induces proliferation of endothelial cells. In mice with CVB3-induced myocarditis, an increase in the number of proliferating endothelial cells was found within the myocardium, which increased 3 days after infection, peaked at day 7, and finally decreased at day 21. [63] Unclear is whether this increase in proliferating endothelial cells in this study is associated with angiogenesis or re-epithelization. [63] Nevertheless, in mice with CVB3-, CVB4-, and encephalomyocarditis virus-induced myocarditis, narrowed capillary lumens have been observed [64, 65], which may be an explanation for the observed endothelial cell proliferation. Next to endothelial cell proliferation, T. cruzi infection has also been shown to stimulate proliferation of vascular smooth muscle cells in vitro. [66]
Induction of thrombosis
The putative infection, pro-inflammatory activation, and death of cardiac endothelial cells each create a potential procoagulant environment. Indeed, occlusive thrombi, fibrin deposits, and aggregated platelets have been found in the small epicardial and intramyocardial vasculature of T. cruzi-infected mice and dogs and in mice with CVB3-induced myocarditis. [67, 68] Such microvascular occlusions could provoke local ischemia in the myocardium as has been suggested to occur in Chagas myocarditis. [69] In addition, mural thrombi (i.e., thrombi that formed on the endocardium) have been found in patients with Chagas disease or lymphocytic myocarditis (Fig. 1b). [70, 71] Similarly, mural thrombi have been observed in mice with CBV3-, encephalomyocarditis virus-, or T. cruzi-induced myocarditis. [68, 72,73,74] Moreover, in mice with CVB3-induced myocarditis, the chance of mural thrombi increased over time. [73] Such mural thrombi pose a clear danger for thromboembolism.
In the hearts of mice with CVB3-induced myocarditis and in EMB of patients with suspected inflammatory cardiomyopathy, an increase in tissue factor (TF) expression was shown. [72] TF is the key initiator of the extrinsic pathway of coagulation. [75] Cardiac TF normally is expressed on cardiomyocytes and is important for maintaining homeostasis in the heart and prevents against spontaneous cardiac hemorrhage. [76] However, in mice with CVB3-induced myocarditis, TF was found to be expressed also on the surface of the cardiac microvasculature, coinciding with fibrin deposits [72], underscoring that the luminal surface of the cardiac microvasculature can become intently procoagulant in infectious myocarditis. Nevertheless, whether cardiac microvascular TF expression occurs on endothelial cells or on subendothelial tissue that becomes exposed after endothelial damage, or both, is not certain yet. Cardiac microvascular endothelial TF expression has been observed in the hearts of patients with myocardial infarction [77], indicating that cardiac microvascular endothelial cells can express TF. Moreover, infection of human umbilical vein endothelial cells with myocarditis-associated viruses in vitro has been shown to increase TF expression and blood clotting. [78, 79] In addition, pro-inflammatory cytokines such as TNFα and IL6 were shown to increase the expression of TF as well as soluble TF in human umbilical vein endothelial cells. [72, 80]
Increased thrombogenicity has also been observed in the blood in infectious myocarditis. In mice with CVB3 myocarditis, increased TF activity was found in the blood [72], and in patients with chronic Chagas disease increased levels of soluble TF and an increased endogenous thrombin potential in the blood have been observed (Fig. 1b). [81] Moreover, platelets isolated from mice infected with T. cruzi were more prone to aggregate than those from uninfected controls. [82] This may be related to an increased production of the eicosanoid thromboxane A2 (TXA2), by platelets and/or endothelial cells, which can amplify platelet activation via autocrine or paracrine pathways. [82, 83] Also, in mice with Chagas disease, elevated levels of TXA2 are found in the plasma. [82, 84] The prothrombotic state may be caused through the damage of the endothelial cells as in rats with acute Chagas disease there is swelling of the endothelial cells and endothelium damage through exposing the subendothelial collagen. [85]
Importantly, the hypercoagulable state that exists in infectious myocarditis appears to be a double-edged sword. On the one hand, excessive clotting can lead to intravascular coagulation and subsequent tissue damage [86], while on the other hand activation of coagulation during infection is a protective mechanism to limit the spread of infectious pathogens. [6] Coagulation proteases, such as thrombin, can activate cells by cleavage of protease-activated receptors (PARs), which can modulate the immune response to a viral infection. [86] In addition, the expression of PARs is increased in endothelial cells after cardiotropic viral infection. [87] In PAR-1-deficient mice, infection with CVB3 led to increased viral loads in the heart and increased cardiac injury compared with wild-type mice [88], pointing to the importance of the TF/thrombin/PAR pathway in anti-viral immunity. Conversely, however, PAR-2-deficient mice had a reduced viral load in the heart and were protected from developing myocarditis. [89] These studies highlight that PARs modulate the innate immune response and can have both positive and negative effects identify that the PAR receptors and thereby the coagulation system plays an important role in the immune response in viral induced myocarditis.
Effects on coronary artery atherosclerosis
Aberrations in the blood vessels of the heart in infectious myocarditis are not limited to the intramyocardial microvasculature but can also occur in the large epicardial coronary arteries and in atherosclerotic plaques therein. [90] A substantial proportion of infectious myocarditis patients have complaints of chest pain with angina-like characteristics that point to coronary artery disease or even myocardial infarction. [91,92,93] Infectious myocarditis patients can present with additional infarct-like symptoms such as ST-segment elevation on ECG, wall motion abnormalities, and elevated circulating cardiac troponin levels. [94, 95] However, despite the similarities in clinical presentation, infectious myocarditis and myocardial infarction are generally considered as distinct clinical entities.
In clinical practice, infectious myocarditis is only considered as the underlying cause of infarct-like complaints when myocardial infarction is ruled out, based on the absence of angiographic coronary narrowing or obstruction. In case of normal or non-obstructed coronary arteries, use of CMR imaging has been suggested as a complementary imaging tool to further discriminate between myocarditis and myocardial infarction, wherein myocardial injury is mainly located in the subendocardium with myocardial infarction as opposed to a more (sub)epicardial location with myocarditis. [96, 97] Indeed, in 50 to 78% of the patients with acute chest pain but without angiographically determined obstructed coronary arteries, the underlying cause was found to be acute myocarditis. [93, 98, 99] Several observations suggest an interrelatedness between infectious myocarditis and myocardial infarction. Recent respiratory tract influenza virus infections for instance, a virus commonly associated with myocarditis, are significantly associated with the development of myocardial infarction also [100], while vaccination against influenza is associated with a decreased risk of myocardial infarction [101]. In addition, enteroviruses (Coxsackie B viruses) were detected in the hearts of 40% of patients who died of sudden myocardial infarction versus only 4% of matched subjects without cardiac disease. [102] 103] Moreover, one case report demonstrated the co-occurrence of EMB-confirmed parvovirus B19-related myocarditis and acute myocardial infarction caused by a thrombus-occluded epicardial coronary artery (Fig. 2). [104] Another case with myocardial infarction due to coronary thrombosis was reported, wherein enterovirus was detected postmortem both in the coronary atherosclerotic plaque and in heart tissue. [105] Similarly, in a third case, presumed myopericarditis co-occurred with coronary angiography and CMR proven acute posterior myocardial infarction, related to a right coronary artery thrombosis, and a concurrent Coxsackievirus B2 infection. [106] Furthermore, we have recently shown in autopsied cases of patients diagnosed postmortem with lymphocytic myocarditis a high prevalence of very recent myocardial infarction, some with thrombus-occluded coronary arteries. [90] These data demonstrate that infectious myocarditis and myocardial infarction can be present simultaneously, also in patients with occluded epicardial coronary arteries. Even more, these data suggest that infectious myocarditis either via a direct virus effect or indirectly via the activated immune response and inflammation can facilitate the development of myocardial infarction.
Myocardial infarction most often is the result of coronary atherosclerotic plaque complication, either through rupture or erosion of plaques, followed by thrombus formation. Intraplaque inflammation is an important mediator of atherosclerotic plaque destabilization that renders them more vulnerable for complication. [107] We observed increased intraplaque infiltration of macrophages and neutrophils, increased intraplaque hemorrhage, and increased instability in coronary artery atherosclerotic plaques of autopsied lymphocytic myocarditis patients [90] (Fig. 2), indicating that infectious myocarditis may facilitate myocardial infarction through an effect on atherosclerotic plaques.
In theory, these effects could be the result of direct infection of coronary artery plaques. As many cardiotropic viruses have been shown to infect endothelial cells (Supplementary Table 1), they may also do so in the epicardial coronary arteries and coronary atherosclerotic plaques [108,109,110,111,112]. In fact, a wide range of pathogens, including cardiotropic viruses, have been identified by genome or antigen detection methods in human atherosclerotic plaques [108], although, to the best of our knowledge, infection of atherosclerotic lesions with T. cruzi has never been reported. The cardiotropic viruses cytomegalovirus [109,110,111], herpes simplex virus [110] enterovirus (echovirus 9 and coxsackieviruses B1 and B3) [109], Epstein-Barr virus [110], and parvovirus B19 [112] have been found in human coronary atherosclerotic plaques. In fact, enhanced cytomegalovirus infection was found in human atherosclerotic vessels compared to non-atherosclerotic vessels [111], suggesting that atherosclerotic plaques have increased susceptibility for viral infection. However, it has to be noticed that in these studies concomitant myocarditis was not reported. Although the precise mechanisms are not exactly known, it has become clear from studies in animal models that viral infection can induce or accelerate atherosclerosis. In 1978, it was shown in chickens that infection with Marek’s disease herpesvirus, either or not in combination with a high fat diet, resulted in grossly visible atherosclerotic lesions in large coronary arteries and aortas, while these were absent in uninfected chickens. [113] Moreover, in atherosclerotic ApoE−/− mice, influenza A has been reported to directly infect aortic atherosclerotic lesions, as well as the heart. [114] This resulted in higher levels of chemokines and cytokines in the blood and the plaque as well as a higher density of intraplaque macrophages. Interestingly, intraplaque inflammation and thrombotic effects were also observed in ApoE−/− mice infected with influenza A, but without evidence of direct infection of the plaques. [115]. It thus seems that infection with influenza, or other cardiotropic viruses, might enhance the chance of a myocardial infarction through increased plaque inflammation and resultant plaque complication, whether or not through direct plaque infection, via increased inflammation in the blood or in the heart and a prothrombotic state. [101] However, such mechanism remains to be proven.
Alternatively, the cardiac and systemic inflammation associated with infectious myocarditis may by itself affect atherosclerotic plaques, irrespective of the infectious pathogens. Indeed, studies have shown that different diseases characterized by increased systemic or cardiac inflammation, including rheumatoid arthritis, vasculitis, and myocardial infarction itself, can accelerate atherosclerosis. [116,117,118] However, limited studies of the context of atherosclerosis are known in patients with Chagas disease. Nonetheless, microscopy revealed that patients with chronic Chagas disease had morphological similar atherosclerosis as patients without Chagas disease. [119] Moreover, patients with and without Chagas disease presenting a myocardial infarction had a comparable frequency of angiographically normal coronary arteries. [120] Nevertheless, there are a few reports that do show that Chagas disease can coincide with myocardial infarction. [121,122,123]
Effects on coronary vasomotor function
Patients with myocarditis frequently report with chest pain complaints, often in the absence of significant coronary obstructive artery disease. [124, 125] These angina pectoris-like symptoms are likely caused by an aberrant vasomotor function of the coronary vasculature. This may be the result of reduced vasodilator capacity and/or hyper-constriction (spasm) of coronary (micro)vasculature. Coronary artery spasm has been frequently reported in patients with viral myocarditis (Fig. 2). [126,127,128] Already in 1985 vascular spasm in the arterioles and capillaries in mice with Chagas disease was reported. [129]
An often used technique to determine whether coronary arteries are sensitive to vasospasm is to expose the coronary arteries to acetylcholine, which can have a direct vasoconstricting action on the vascular smooth muscle provoking coronary vasospasm, and to analyze the vascular responses by angiography. [128] In addition, acetylcholine may also affect the vasodilating action mediated by the endothelium of the coronaries. [130] In patients with Chagas disease and patients with EMB-proven lymphocytic myocarditis, impaired endothelium-dependent impaired coronary vasodilation was demonstrated in the presence of acetylcholine, which may reflect abnormal endothelial function. [53, 131] Similarly, decreased acetylcholine vasodilation was also found in the coronary arteries of mice with CVB3-induced chronic myocarditis. [132]
The underlying cause of vasospasm in patients with infectious myocarditis may relate to presence of the infection itself. Of patients with inflammatory cardiomyopathy, it is known that the endothelial function is impaired in patients with virus persistence compared to patients without virus. [133] Moreover, the endothelial dysfunction was more pronounced in patients that showed increased inflammation in the myocardium. In addition, in patients with clinical myocardial infarction, but with angiographically normal coronary arteries, viral genome could be detected in 71% of the EMB, mainly parvovirus B19, but also enterovirus and adenovirus. [92] Likewise, in patients with clinically suspected myocarditis, exposing the coronary arteries to acetylcholine resulted in coronary vasospasm in 70.9%. [128] Of these patients, the ones with proven parvovirus B19 myocarditis had significantly more often coronary vasospasm than patients without myocardial inflammation and/or virus. [128] These studies suggest that virus infection can interfere with the endothelial function of the coronary arteries.
Furthermore, the initiation of vasospasm and/or endothelial dysfunction may also relate to the increased inflammation within the myocardium in patients with infectious myocarditis (Fig. 2). In patients with lymphocytic myocarditis, a correlation was noted between the number of lymphocytes in the myocardium and the epicardial coronary constriction in response to acetylcholine. [53] In patients with myocarditis also, a correlation between impaired endothelial function, determined by flow-mediated vasodilation in the peripheral radial arteries, and enhanced endothelial expression of MHC and adhesion molecules in EMB was observed. [132] Furthermore, coronary vasospasm was found to occur often at the site of atherosclerotic lesions which are associated with impaired endothelium-dependent vasodilation. [130, 134] These studies indeed suggest that coronary vasospasm is associated with inflammation in the heart.
The occurrence of coronary vasospasm in patients with infectious myocarditis may relate to vasoactive substances, like the powerful vasoconstrictors Endothelin-1 (ET-1) and TXA2 [69, 135] (Fig. 2). In humans with chronic Chagas disease, increased plasma levels of ET-1 have been demonstrated. [136] Similarly, also in mice with Chagas disease or viral myocarditis, elevated levels of ET-1 in the plasma have also been observed, but also increased expression of ET-1 on the endothelium of the coronary arteries. [137,138,139] Elevated levels of TXA2 in the plasma and increased gene expression of thromboxane synthase in the myocardium were demonstrated in mice with T. cruzi-induced Chagas disease. [80, 84] Interestingly, T. cruzi is also capable of synthesizing TXA2 itself and the majority of TXA2 in the blood of infected mice is derived from the parasite. [140]
We have observed in autopsied patients with lymphocytic myocarditis elevated intraplaque hemorrhage and mast cells in the epicardial coronary arteries. [90] A possible cause of intraplaque hemorrhage is coronary vasospasm. Interestingly, via the secretion of vasoactive factors such as histamine, chymase, and tryptase, mast cells have also been associated with coronary vasospasm. [141]
Effects on cardiac perfusion
The above described prothrombotic, pro-atherosclerotic, and vasospastic effects can in theory result in impaired perfusion of the heart, resulting in (transient) cardiac ischemia. [8] One important parameter to assess whether the functional and structural abnormalities of the cardiac vessels interfere with the blood circulation is the coronary flow reserve, i.e., the degree of the increase in coronary flow from the basal to the maximal coronary vasodilation. Reduced coronary flow reserve indicates the presence of either a flow-limiting coronary artery stenosis and/or dysfunction of the coronary microcirculation. [142, 143] Impaired coronary flow reserve has been observed in patients with viral myocarditis and in patients with acute Chagas disease [144,145,146,147,148], but also patients with chronic Chagas disease [69, 147, 148]. The impaired coronary flow reserve in acute Chagas patients has been correlated with age, severity of Chagas, and ventricular heart function. [142, 146] This decreased cardiac perfusion in Chagas disease was observed in patients with angiographically normal coronary arteries, supporting the concept that abnormal flow regulation essentially occurs at the microvascular level. [142, 144,145,146] Similarly, in patients with viral myocarditis mimicking acute coronary syndrome with angiographically normal coronary arteries, a reduced coronary flow was observed up to 1 year after hospital administration compared to healthy participants. The coronary flow was especially reduced during the first week and recovered with time. [149]
The presence of virus in the heart may affect the cardiac perfusion, since in patients with EMB-proven myocarditis the coronary blood flow was found to be significantly impaired when myocardial virus persisted compared to absence of virus detection. [150] In mice with CVB3-induced myocarditis, the coronary flow reserve was significantly reduced 1 week after CVB3 infection and was still impaired 2 weeks after infection and correlated with the severity of myocarditis. [143] Moreover, in mice with encephalomyocarditis virus-induced myocarditis, a decreased perfusion of the small intramyocardial arteries has been observed. [151] Also, mice infected with T. cruzi had decreased microcirculatory flow within the arterioles and venules. The arterioles of these infected mice exhibited segmental areas of vasospasm and dilatation. [152]
Conclusion
In this review, we have provided an overview of the functional and structural changes in the coronary (micro)vasculature that occur in infectious myocarditis and the effects that these changes have or may have on its pathogenesis. Clearly, the coronary (micro)vasculature plays a prominent role in the different stages of the disease; initially as a barrier against and as a target for infection, and subsequently as an important factor in the shaping of the immune response in the heart and as an important determinant of dysfunction of the heart. As such, these changes in the coronary (micro)vasculature may explain, in part, the wide variety of clinical symptoms in infectious myocarditis patients. Moreover, evidence is accumulating that infectious myocarditis can co-occur with myocardial infarction and that it may facilitate its development via destabilization of atherosclerotic plaques. Given that infectious myocarditis is believed to be clinically silent in most cases, it may be an undetected underlying cause in patients that present with myocardial infarction. However, more research is needed to determine the clinical and prognostic implications of disease co-occurrence.
References
Biesbroek PS, Beek AM, Germans T, Niessen HW, van Rossum AC (2015) Diagnosis of myocarditis: current state and future perspectives. Int J Cardiol 191:211–219
Baksi AJ, Kanaganayagam GS, Prasad SK (2015) Arrhythmias in viral myocarditis and pericarditis. Card Electrophysiol Clin 7:269–281
Theleman KP, Kuiper JJ, Roberts WC (2001) Acute myocarditis (predominately lymphocytic) causing sudden death without heart failure. Am J Cardiol 88:1078–1083
Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB et al (2013) Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 34:2636–2648 48a-48d
Esper L, Talvani A, Pimentel P, Teixeira MM, Machado FS (2015) Molecular mechanisms of myocarditis caused by Trypanosoma cruzi. Curr Opin Infect Dis 28:246–252
Antoniak S, Mackman N (2014) Coagulation, protease-activated receptors, and viral myocarditis. J Cardiovasc Transl Res 7:203–211
Liu PP, Mason JW (2001) Advances in the understanding of myocarditis. Circulation 104:1076–1082
Blauwet LA, Cooper LT (2010) Myocarditis. Prog Cardiovasc Dis 52:274–288
Teixeira AR, Hecht MM, Guimaro MC, Sousa AO, Nitz N (2011) Pathogenesis of Chagas’ disease: parasite persistence and autoimmunity. Clin Microbiol Rev 24:592–630
Rassi A Jr, Rassi A, Marin-Neto JA (2010) Chagas disease. Lancet 375:1388–1402
Marin-Neto JA, Cunha-Neto E, Maciel BC, Simoes MV (2007) Pathogenesis of chronic Chagas heart disease. Circulation 115:1109–1123
Bracamonte-Baran W, Cihakova D (2017) Cardiac autoimmunity: myocarditis. Adv Exp Med Biol 1003:187–221
Iwasaki T, Monma N, Satodate R, Segawa I, Oyama K, Kawana R, Kurata T (1985) Myocardial lesions by Coxsackie virus B3 and cytomegalovirus infection in infants. Heart Vessels Suppl 1:167–172
Salgado DM, Eltit JM, Mansfield K, Panqueba C, Castro D, Vega MR, Xhaja K, Schmidt D, Martin KJ, Allen PD, Rodriguez JA, Dinsmore JH, López JR, Bosch I (2010) Heart and skeletal muscle are targets of dengue virus infection. Pediatr Infect Dis J 29:238–242
Povoa TF, Alves AM, Oliveira CA, Nuovo GJ, Chagas VL, Paes MV (2014) The pathology of severe dengue in multiple organs of human fatal cases: histopathology, ultrastructure and virus replication. PLoS One 9:e83386
Saggioro FP, Rossi MA, Duarte MI, Martin CC, Alves VA, Moreli ML et al (2007) Hantavirus infection induces a typical myocarditis that may be responsible for myocardial depression and shock in hantavirus pulmonary syndrome. J Infect Dis 195:1541–1549
Leveque N, Boulagnon C, Brasselet C, Lesaffre F, Boutolleau D, Metz D, Fornes P, Andreoletti L (2011) A fatal case of Human Herpesvirus 6 chronic myocarditis in an immunocompetent adult. J Clin Virol 52:142–145
Bultmann BD, Klingel K, Sotlar K, Bock CT, Baba HA, Sauter M et al (2003) Fatal parvovirus B19-associated myocarditis clinically mimicking ischemic heart disease: an endothelial cell-mediated disease. Hum Pathol 34:92–95
Higuchi Mde L, De Brito T, Martins Reis M, Barbosa A, Bellotti G, Pereira-Barreto AC et al (1993) Correlation between Trypanosoma cruzi parasitism and myocardial inflammatory infiltrate in human chronic chagasic myocarditis: light microscopy and immunohistochemical findings. Cardiovasc Pathol 2:101–106
Vlemmas J, Billinis C, Psychas V, Papaioannou N, Paschaleri-Papadopoulou E, Leontides S, Papadopoulos O (2000) Immunohistochemical detection of encephalomyocarditis virus (EMCV) antigen in the heart of experimentally infected piglets. J Comp Pathol 122:235–240
Burch GE, Rayburn P (1977) EMC viral infection of the coronary blood vessels in newborn mice: viral vasculitis. Br J Exp Pathol 58:565–571
Machida N, Taniguchi T, Nakamura T, Kiryu K (1997) Cardio-histopathological observations on aborted equine fetuses infected with equid herpesvirus 1 (EHV-1). J Comp Pathol 116:379–385
Rodriguez HO, Guerrero NA, Fortes A, Santi-Rocca J, Girones N, Fresno M (2014) Trypanosoma cruzi strains cause different myocarditis patterns in infected mice. Acta Trop 139:57–66
Bachelier K, Biehl S, Schwarz V, Kindermann I, Kandolf R, Sauter M, Ukena C, Yilmaz A, Sliwa K, Bock CT, Klingel K, Böhm M (2017) Parvovirus B19-induced vascular damage in the heart is associated with elevated circulating endothelial microparticles. PLoS One 12:e0176311
Ino T, Kishiro M, Okubo M, Akimoto K, Nishimoto K, Yabuta K, Okada R (1997) Late persistent expressions of ICAM-1 and VCAM-1 on myocardial tissue in children with lymphocytic myocarditis. Cardiovasc Res 34:323–328
Benvenuti LA, Higuchi ML, Reis MM (2000) Upregulation of adhesion molecules and class I HLA in the myocardium of chronic chagasic cardiomyopathy and heart allograft rejection, but not in dilated cardiomyopathy. Cardiovasc Pathol 9:111–117
Baidoshvili A, Krijnen PA, Kupreishvili K, Ciurana C, Bleeker W, Nijmeijer R et al (2006) N(epsilon)-(carboxymethyl)lysine depositions in intramyocardial blood vessels in human and rat acute myocardial infarction: a predictor or reflection of infarction? Arterioscler Thromb Vasc Biol 26:2497–2503
Marino AP, Azevedo MI, Lannes-Vieira J (2003) Differential expression of adhesion molecules shaping the T-cell subset prevalence during the early phase of autoimmune and Trypanosoma cruzi-elicited myocarditis. Mem Inst Oswaldo Cruz 98:945–952
McDouall RM, Farrar MW, Khan S, Yacoub MH, Allen SP (2001) Unique sensitivities to cytokine regulated expression of adhesion molecules in human heart-derived endothelial cells. Endothelium 8:25–40
Keating SM, Deng X, Fernandes F, Cunha-Neto E, Ribeiro AL, Adesina B, Beyer AI, Contestable P, Custer B, Busch MP, Sabino EC, NHLBI Retrovirus Epidemiology Donor Study-II (REDS-II), International Component (2015) Inflammatory and cardiac biomarkers are differentially expressed in clinical stages of Chagas disease. Int J Cardiol 199:451–459
Anisuzzaman HT, Miyoshi T, Matsubayashi M, Islam MK, Alim MA et al (2014) Longistatin in tick saliva blocks advanced glycation end-product receptor activation. J Clin Invest 124:4429–4444
Yu Y, Yu Y, Liu M, Yu P, Liu G, Liu Y, Su Y, Jiang H, Chen R (2016) Ethyl pyruvate attenuated coxsackievirus B3-induced acute viral myocarditis by suppression of HMGB1/RAGE/NF-KappaB pathway. Spring 5:215
Zanone MM, Favaro E, Conaldi PG, Greening J, Bottelli A, Perin PC, Klein NJ, Peakman M, Camussi G (2003) Persistent infection of human microvascular endothelial cells by coxsackie B viruses induces increased expression of adhesion molecules. J Immunol 171:438–446
Shen J, T-To SS, Schrieber L, King NJ (1997) Early E-selectin, VCAM-1, ICAM-1, and late major histocompatibility complex antigen induction on human endothelial cells by flavivirus and comodulation of adhesion molecule expression by immune cytokines. J Virol 71:9323–9332
Xiao C, Bator CM, Bowman VD, Rieder E, He Y, Hebert B, Bella J, Baker TS, Wimmer E, Kuhn RJ, Rossmann MG (2001) Interaction of coxsackievirus A21 with its cellular receptor, ICAM-1. J Virol 75:2444–2451
Evans DJ, Almond JW (1998) Cell receptors for picornaviruses as determinants of cell tropism and pathogenesis. Trends Microbiol 6:198–202
Kuhl U, Noutsias M, Seeberg B, Schultheiss HP (1996) Immunohistological evidence for a chronic intramyocardial inflammatory process in dilated cardiomyopathy. Heart 75:295–300
Hufnagel G, Maisch B (1991) Expression of MHC class I and II antigens and the Il-2 receptor in rejection, myocarditis and dilated cardiomyopathy. Eur Heart J 12(Suppl D):137–140
Reis DD, Jones EM, Tostes S, Lopes ER, Chapadeiro E, Gazzinelli G et al (1993) Expression of major histocompatibility complex antigens and adhesion molecules in hearts of patients with chronic Chagas’ disease. Am J Trop Med Hyg 49:192–200
Li W, Yu S, Zhao H (1995) Expression of MHC-I and MHC-II antigens in endomyocardial biopsies from patients with viral myocarditis and cardiomyopathy. Chin Med J 108:809–811
Kumar A, Perdomo MF, Kantele A, Hedman L, Hedman K, Franssila R (2015) Granzyme B mediated function of Parvovirus B19-specific CD4(+) T cells. Clin Transl Immunol 4:e39
Morrison LA, Lukacher AE, Braciale VL, Fan DP, Braciale TJ (1986) Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med 163:903–921
Green S, Kurane I, Pincus S, Paoletti E, Ennis FA (1997) Recognition of dengue virus NS1-NS2a proteins by human CD4+ cytotoxic T lymphocyte clones. Virology 234:383–386
Penna A, Fowler P, Bertoletti A, Guilhot S, Moss B, Margolskee RF, Cavalli A, Valli A, Fiaccadori F, Chisari FV (1992) Hepatitis B virus (HBV)-specific cytotoxic T-cell (CTL) response in humans: characterization of HLA class II-restricted CTLs that recognize endogenously synthesized HBV envelope antigens. J Virol 66:1193–1198
Munz C, Bickham KL, Subklewe M, Tsang ML, Chahroudi A, Kurilla MG et al (2000) Human CD4(+) T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J Exp Med 191:1649–1660
Chaplin DD (2010) Overview of the immune response. J Allergy Clin Immunol 125:S3–23
McCarthy MK, Procario MC, Twisselmann N, Wilkinson JE, Archambeau AJ, Michele DE et al (2015) Proinflammatory effects of interferon gamma in mouse adenovirus 1 myocarditis. J Virol 89:468–479
Ferreira LR, Frade AF, Baron MA, Navarro IC, Kalil J, Chevillard C, Cunha-Neto E (2014) Interferon-gamma and other inflammatory mediators in cardiomyocyte signaling during Chagas disease cardiomyopathy. World J Cardiol 6:782–790
Thelemann C, Haller S, Blyszczuk P, Kania G, Rosa M, Eriksson U, Rotman S, Reith W, Acha-Orbea H (2016) Absence of nonhematopoietic MHC class II expression protects mice from experimental autoimmune myocarditis. Eur J Immunol 46:656–664
Taneja V, David CS (2009) Spontaneous autoimmune myocarditis and cardiomyopathy in HLA-DQ8.NODAbo transgenic mice. J Autoimmun 33:260–269
Leipner C, Borchers M, Merkle I, Stelzner A (1999) Coxsackievirus B3-induced myocarditis in MHC class II-deficient mice. J Hum Virol 2:102–114
Baessler B, Schaarschmidt F, Dick A, Stehning C, Schnackenburg B, Michels G et al (2015) Mapping tissue inhomogeneity in acute myocarditis: a novel analytical approach to quantitative myocardial edema imaging by T2-mapping. J Cardiovasc Magn Reson 17:115
Klein RM, Schwartzkopff B, Strauer BE (1998) Evidence of endothelial dysfunction of epicardial coronary arteries in patients with immunohistochemically proven myocarditis. Am Heart J 136:389–397
Lum H, Malik AB (1996) Mechanisms of increased endothelial permeability. Can J Physiol Pharmacol 74:787–800
Svensjo E, Cyrino F, Michoud E, Ruggiero D, Bouskela E, Wiernsperger N (1999) Vascular permeability increase as induced by histamine or bradykinin is enhanced by advanced glycation endproducts (AGEs). J Diabetes Complicat 13:187–190
Xie Y, Liao J, Li M, Wang X, Yang Y, Ge J, Chen R, Chen H (2012) Impaired cardiac microvascular endothelial cells function induced by Coxsackievirus B3 infection and its potential role in cardiac fibrosis. Virus Res 169:188–194
Lander HM, Grant AM, Albrecht T, Hill T, Peters CJ (2014) Endothelial cell permeability and adherens junction disruption induced by junin virus infection. Am J Trop Med Hyg 90:993–1002
Ju Y, Wang T, Li Y, Xin W, Wang S, Li J (2007) Coxsackievirus B3 affects endothelial tight junctions: possible relationship to ZO-1 and F-actin, as well as p38 MAPK activity. Cell Biol Int 31:1207–1213
Raekiansyah M, Espada-Murao LA, Okamoto K, Kubo T, Morita K (2014) Dengue virus neither directly mediates hyperpermeability nor enhances tumor necrosis factor-alpha-induced permeability in vitro. Jpn J Infect Dis 67:86–94
Andrade D, Serra R, Svensjo E, Lima AP, Ramos ES Jr, Fortes FS et al (2012) Trypanosoma cruzi invades host cells through the activation of endothelin and bradykinin receptors: a converging pathway leading to chagasic vasculopathy. Br J Pharmacol 165:1333–1347
Novaes RD, Penitente AR, Goncalves RV, Talvani A, Peluzio MC, Neves CA et al (2013) Trypanosoma cruzi infection induces morphological reorganization of the myocardium parenchyma and stroma, and modifies the mechanical properties of atrial and ventricular cardiomyocytes in rats. Cardiovasc Pathol 22:270–279
Higuchi ML, Fukasawa S, De Brito T, Parzianello LC, Bellotti G, Ramires JA (1999) Different microcirculatory and interstitial matrix patterns in idiopathic dilated cardiomyopathy and Chagas’ disease: a three dimensional confocal microscopy study. Heart 82:279–285
Zhao L, Li S, Ge J, Sun A, Zou Y, Zhang S (2008) Temporal changes in stem cells in the circulation and myocardium of mice with Coxsackie virus B3-induced myocarditis. Microvasc Res 75:358–366
Burch GE (1975) The coronary arteries in active viral cardiomyopathies. Postgrad Med J 51:311–324
Silver MA, Kowalczyk D (1989) Coronary microvascular narrowing in acute murine coxsackie B3 myocarditis. Am Heart J 118:173–174
Hassan GS, Mukherjee S, Nagajyothi F, Weiss LM, Petkova SB, de Almeida CJ, Huang H, Desruisseaux MS, Bouzahzah B, Pestell RG, Albanese C, Christ GJ, Lisanti MP, Tanowitz HB (2006) Trypanosoma cruzi infection induces proliferation of vascular smooth muscle cells. Infect Immun 74:152–159
Rossi MA, Goncalves S, Ribeiro-dos-Santos R (1984) Experimental Trypanosoma cruzi cardiomyopathy in BALB/c mice. The potential role of intravascular platelet aggregation in its genesis. Am J Pathol 114:209–216
Andrade ZA, Andrade SG, Correa R, Sadigursky M, Ferrans VJ (1994) Myocardial changes in acute Trypanosoma cruzi infection. Ultrastructural evidence of immune damage and the role of microangiopathy. Am J Pathol 144:1403–1411
Prado CM, Jelicks LA, Weiss LM, Factor SM, Tanowitz HB, Rossi MA (2011) The vasculature in chagas disease. Adv Parasitol 76:83–99
Kojima J, Miyazaki S, Fujiwara H, Kumada T, Kawai C (1988) Recurrent left ventricular mural thrombi in a patient with acute myocarditis. Heart Vessel 4(2):120–122
Okabe M, Fukuda K, Nakashima Y, Hiroki T, Arakawa K, Kikuchi M (1992) Lymphocytic active myocarditis characterized by numerous clusters of lymphocytes: a chronic variant of myocarditis? Am Heart J 123:128–136
Antoniak S, Boltzen U, Riad A, Kallwellis-Opara A, Rohde M, Dorner A et al (2008) Viral myocarditis and coagulopathy: increased tissue factor expression and plasma thrombogenicity. J Mol Cell Cardiol 45:118–126
Kishimoto C, Ochiai H, Sasayama S (1992) Intracardiac thrombus in murine Coxsackievirus B3 myocarditis. Heart Vessel 7:76–81
Tomioka N, Kishimoto C, Matsumori A, Kawai C (1986) Mural thrombus in experimental viral myocarditis in mice: relation between thrombosis and congestive heart failure. Cardiovasc Res 20:665–671
Bode MF, Mackman N (2015) Protective and pathological roles of tissue factor in the heart. Hamostaseologie 35:37–46
Pawlinski R, Tencati M, Holscher T, Pedersen B, Voet T, Tilley RE et al (2007) Role of cardiac myocyte tissue factor in heart hemostasis. J Thromb Haemost 5:1693–1700
Krijnen PA, Hahn NE, Kholova I, Baylan U, Sipkens JA, van Alphen FP et al (2012) Loss of DPP4 activity is related to a prothrombogenic status of endothelial cells: implications for the coronary microvasculature of myocardial infarction patients. Basic Res Cardiol 107:233
Visseren FL, Bouwman JJ, Bouter KP, Diepersloot RJ, de Groot PH, Erkelens DW (2000) Procoagulant activity of endothelial cells after infection with respiratory viruses. Thromb Haemost 84:319–324
Key NS, Vercellotti GM, Winkelmann JC, Moldow CF, Goodman JL, Esmon NL, Esmon CT, Jacob HS (1990) Infection of vascular endothelial cells with herpes simplex virus enhances tissue factor activity and reduces thrombomodulin expression. Proc Natl Acad Sci U S A 87:7095–7099
Antoniak S, Boltzen U, Eisenreich A, Stellbaum C, Poller W, Schultheiss HP et al (2009) Regulation of cardiomyocyte full-length tissue factor expression and microparticle release under inflammatory conditions in vitro. J Thromb Haemost 7:871–878
Herrera RN, Diaz de Amaya EI, Perez Aguilar RC, Joo Turoni C, Maranon R, Berman SG et al (2011) Inflammatory and prothrombotic activation with conserved endothelial function in patients with chronic, asymptomatic Chagas disease. Clin Appl Thromb Hemost 17:502–507
Tanowitz HB, Burns ER, Sinha AK, Kahn NN, Morris SA, Factor SM et al (1990) Enhanced platelet adherence and aggregation in Chagas’ disease: a potential pathogenic mechanism for cardiomyopathy. Am J Trop Med Hyg 43:274–281
Ramadan FM, Upchurch GR Jr, Keagy BA, Johnson G Jr (1990) Endothelial cell thromboxane production and its inhibition by a calcium-channel blocker. Ann Thorac Surg 49:916–919
Molina-Berrios A, Campos-Estrada C, Lapier M, Duaso J, Kemmerling U, Galanti N et al (2013) Benznidazole prevents endothelial damage in an experimental model of Chagas disease. Acta Trop 127:6–13
Rossi MA (1997) Aortic endothelial cell changes in the acute septicemic phase of experimental Trypanosoma cruzi infection in rats: scanning and transmission electron microscopic study. Am J Trop Med Hyg. 57:321–327
Antoniak S, Mackman N (2014) Multiple roles of the coagulation protease cascade during virus infection. Blood 123:2605–2613
Popovic M, Paskas S, Zivkovic M, Burysek L, Laumonnier Y (2010) Human cytomegalovirus increases HUVEC sensitivity to thrombin and modulates expression of thrombin receptors. J Thromb Thrombolysis 30:164–171
Antoniak S, Owens AP 3rd, Baunacke M, Williams JC, Lee RD, Weithauser A et al (2013) PAR-1 contributes to the innate immune response during viral infection. J Clin Invest 123:1310–1322
Weithauser A, Bobbert P, Antoniak S, Bohm A, Rauch BH, Klingel K et al (2013) Protease-activated receptor-2 regulates the innate immune response to viral infection in a coxsackievirus B3-induced myocarditis. J Am Coll Cardiol 62:1737–1745
Woudstra L, Biesbroek PS, Emmens RW, Heymans S, Juffermans LJ, van Rossum AC, Niessen HWM, Krijnen PAJ (2017) Lymphocytic myocarditis occurs with myocardial infarction and coincides with increased inflammation, hemorrhage and instability in coronary artery atherosclerotic plaques. Int J Cardiol 232:53–62
McCully RB, Cooper LT, Schreiter S (2005) Coronary artery spasm in lymphocytic myocarditis: a rare cause of acute myocardial infarction. Heart 91:202
Kuhl U, Pauschinger M, Bock T, Klingel K, Schwimmbeck CP, Seeberg B et al (2003) Parvovirus B19 infection mimicking acute myocardial infarction. Circulation 108:945–950
Sarda L, Colin P, Boccara F, Daou D, Lebtahi R, Faraggi M, Nguyen C, Cohen A, Slama MS, Steg PG, Le Guludec D (2001) Myocarditis in patients with clinical presentation of myocardial infarction and normal coronary angiograms. J Am Coll Cardiol 37:786–792
Amoruso M, Muzzarelli S, Moccetti T, Pedrazzini G (2015) Fulminant lymphocytic myocarditis mimicking ST-elevation myocardial infarction. Eur Heart J 36:2227
Nucifora G, Miani D, Di Chiara A, Piccoli G, Artico J, Puppato M et al (2013) Infarct-like acute myocarditis: relation between electrocardiographic findings and myocardial damage as assessed by cardiac magnetic resonance imaging. Clin Cardiol 36:146–152
Friedrich MG, Marcotte F (2013) Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging 6:833–839
Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P, International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis (2009) Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol 53:1475–1487
Assomull RG, Lyne JC, Keenan N, Gulati A, Bunce NH, Davies SW, Pennell DJ, Prasad SK (2007) The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J 28:1242–1249
Laraudogoitia Zaldumbide E, Perez-David E, Larena JA, Velasco del Castillo S, Rumoroso Cuevas JR, Onaindia JJ et al (2009) The value of cardiac magnetic resonance in patients with acute coronary syndrome and normal coronary arteries. Rev Esp Cardiol 62:976–983
Barnes M, Heywood AE, Mahimbo A, Rahman B, Newall AT, Macintyre CR (2015) Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart 101:1738–1747
MacIntyre CR, Mahimbo A, Moa AM, Barnes M (2016) Influenza vaccine as a coronary intervention for prevention of myocardial infarction. Heart 102:1953–1956
Andreoletti L, Venteo L, Douche-Aourik F, Canas F, Lorin dG, Jacques J et al (2007) Active Coxsackieviral B infection is associated with disruption of dystrophin in endomyocardial tissue of patients who died suddenly of acute myocardial infarction. J Am Coll Cardiol 50:2207–2214
Roivainen M, Alfthan G, Jousilahti P, Kimpimaki M, Hovi T, Tuomilehto J (1998) Enterovirus infections as a possible risk factor for myocardial infarction. Circulation 98:2534–2537
Gutersohn A, Zimmermann U, Bartel T, Erbel R (2005) A rare case of acute ‘infective’ myocardial infarction triggered by acute parvovirus B19 myocarditis. Nat Clin Pract Cardiovasc Med 2:167–171
Calabrese F, Basso C, Valente M, Thiene G (2003) Coronary thrombosis and sudden death after an enteroviral infection. Case report. APMIS 111:315–318
Liapounova NA, Mouquet F, Ennezat PV (2011) Acute myocardial infarction spurred by myopericarditis in a young female patient: Coxsackie B2 to blame. Acta Cardiol 66:79–81
Hansson GK, Libby P, Tabas I (2015) Inflammation and plaque vulnerability. J Intern Med 278:483–493
Rosenfeld ME, Campbell LA (2011) Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb Haemost 106:858–867
Kwon TW, Kim DK, Ye JS, Lee WJ, Moon MS, Joo CH et al (2004) Detection of enterovirus, cytomegalovirus, and Chlamydia pneumoniae in atheromas. J Microbiol 42:299–304
Ibrahim AI, Obeid MT, Jouma MJ, Moasis GA, Al-Richane WL, Kindermann I et al (2005) Detection of herpes simplex virus, cytomegalovirus and Epstein-Barr virus DNA in atherosclerotic plaques and in unaffected bypass grafts. J Clin Virol 32:29–32
Nerheim PL, Meier JL, Vasef MA, Li WG, Hu L, Rice JB, Gavrila D, Richenbacher WE, Weintraub NL (2004) Enhanced cytomegalovirus infection in atherosclerotic human blood vessels. Am J Pathol 164:589–600
Liu SC, Tsai CT, Wu CK, Yu MF, Wu MZ, Lin LI, Wang SS, Hwang JJ, Tseng YZ, Chiang FT, Tseng CD (2009) Human parvovirus b19 infection in patients with coronary atherosclerosis. Arch Med Res 40(7):612–617
Fabricant CG, Fabricant J, Litrenta MM, Minick CR (1978) Virus-induced atherosclerosis. J Exp Med 148:335–340
Haidari M, Wyde PR, Litovsky S, Vela D, Ali M, Casscells SW, Madjid M (2010) Influenza virus directly infects, inflames, and resides in the arteries of atherosclerotic and normal mice. Atherosclerosis 208:90–96
Naghavi M, Wyde P, Litovsky S, Madjid M, Akhtar A, Naguib S, Siadaty MS, Sanati S, Casscells W (2003) Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation 107:762–768
Shoenfeld Y, Gerli R, Doria A, Matsuura E, Cerinic MM, Ronda N, Jara LJ, Abu-Shakra M, Meroni PL, Sherer Y (2005) Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation 112:3337–3347
Cohen Tervaert JW (2013) Cardiovascular disease due to accelerated atherosclerosis in systemic vasculitides. Best Pract Res Clin Rheumatol 27:33–44
Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HWM, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M (2012) Myocardial infarction accelerates atherosclerosis. Nature 487:325–329
Lopes ER, de Mesquita PM, de Mesquita LF, Chapadeiro E (1995) [Coronary arteriosclerosis and myocardial infarction in chronic Chagas’ disease]. Arq Bras Cardiol 65:143–145
Marin-Neto JA, Simoes MV, Ayres-Neto EM, Attab-Santos JL, Gallo L Jr, Amorim DS et al (1995) Studies of the coronary circulation in Chagas’ heart disease. Sao Paulo Med J 113:826–834
Lucchetti G, Granero AL, Caparei FC, Pozzan G, Kadri T, Franken RA (2009) Bilateral papillary muscle infarction in a chagasic patient. Rev Port Cardiol 28:1441–1447
ElMunzer BJ, Sallach SM, McGuire DK (2004) Cardiac chagas disease masquerading as an acute myocardial infarction. Cardiol Rev 12:69–72
De Morais CF, Higuchi ML, Lage S (1989) Chagas’ heart disease and myocardial infarct. Incidence and report of four necropsy cases. Ann Trop Med Parasitol 83:207–214
Leurent G, Langella B, Fougerou C, Lentz PA, Larralde A, Bedossa M, Boulmier D, le Breton H (2011) Diagnostic contributions of cardiac magnetic resonance imaging in patients presenting with elevated troponin, acute chest pain syndrome and unobstructed coronary arteries. Arch Cardiovasc Dis 104:161–170
Shahid M, Hoey E, Basavarajaiah S (2016) Acute myocarditis and ST-segment elevation. Am J Cardiol 118:1605–1608
Kumar A, Bagur R, Beliveau P, Potvin JM, Levesque P, Fillion N et al (2014) Acute myocarditis triggering coronary spasm and mimicking acute myocardial infarction. World J Cardiol 6:1045–1048
Silva D, Marques P, Martins S, Bordalo ESAL, Nobrega J, Duarte J et al (2010) Coronary artery vasospasm and acute myocarditis: a rare association. Rev Port Cardiol 29:1879–1888
Yilmaz A, Mahrholdt H, Athanasiadis A, Vogelsberg H, Meinhardt G, Voehringer M, Kispert EM, Deluigi C, Baccouche H, Spodarev E, Klingel K, Kandolf R, Sechtem U (2008) Coronary vasospasm as the underlying cause for chest pain in patients with PVB19 myocarditis. Heart 94:1456–1463
Factor SM, Cho S, Wittner M, Tanowitz H (1985) Abnormalities of the coronary microcirculation in acute murine Chagas’ disease. Am J Trop Med Hyg 34:246–253
Egashira K, Inou T, Yamada A, Hirooka Y, Takeshita A (1992) Preserved endothelium-dependent vasodilation at the vasospastic site in patients with variant angina. J Clin Invest 89:1047–1052
Torres FW, Acquatella H, Condado JA, Dinsmore R, Palacios IF (1995) Coronary vascular reactivity is abnormal in patients with Chagas’ heart disease. Am Heart J 129:995–1001
Vallbracht KB, Schwimmbeck PL, Seeberg B, Kuhl U, Schultheiss HP (2002) Endothelial dysfunction of peripheral arteries in patients with immunohistologically confirmed myocardial inflammation correlates with endothelial expression of human leukocyte antigens and adhesion molecules in myocardial biopsies. J Am Coll Cardiol 40:515–520
Vallbracht KB, Schwimmbeck PL, Kuhl U, Seeberg B, Schultheiss HP (2004) Endothelium-dependent flow-mediated vasodilation of systemic arteries is impaired in patients with myocardial virus persistence. Circulation 110:2938–2945
Shepherd JT, Vanhoutte PM (1985) Spasm of the coronary arteries: causes and consequences (the scientist’s viewpoint). Mayo Clin Proc 60:33–46
Zaugg CE, Hornstein PS, Zhu P, Simper D, Luscher TF, Allegrini PR, Buser PT (1996) Endothelin-1-induced release of thromboxane A2 increases the vasoconstrictor effect of endothelin-1 in postischemic reperfused rat hearts. Circulation 94:742–747
Salomone OA, Caeiro TF, Madoery RJ, Amuchastegui M, Omelinauk M, Juri D et al (2001) High plasma immunoreactive endothelin levels in patients with Chagas’ cardiomyopathy. Am J Cardiol 87:1217–1220 A7
Ono K, Matsumori A, Shioi T, Furukawa Y, Sasayama S (1999) Contribution of endothelin-1 to myocardial injury in a murine model of myocarditis: acute effects of bosentan, an endothelin receptor antagonist. Circulation 100:1823–1829
Baba T, Kanda T, Kobayashi I (2000) Reduction of cardiac endothelin-1 by angiotensin II type 1 receptor antagonist in viral myocarditis of mice. Life Sci 67:587–597
Petkova SB, Tanowitz HB, Magazine HI, Factor SM, Chan J, Pestell RG, Bouzahzah B, Douglas SA, Shtutin V, Morris SA, Tsang E, Weiss LM, Christ GJ, Wittner M, Huang H (2000) Myocardial expression of endothelin-1 in murine Trypanosoma cruzi infection. Cardiovasc Pathol 9:257–265
Ashton AW, Mukherjee S, Nagajyothi FN, Huang H, Braunstein VL, Desruisseaux MS, Factor SM, Lopez L, Berman JW, Wittner M, Scherer PE, Capra V, Coffman TM, Serhan CN, Gotlinger K, Wu KK, Weiss LM, Tanowitz HB (2007) Thromboxane A2 is a key regulator of pathogenesis during Trypanosoma cruzi infection. J Exp Med 204:929–940
Ozben B, Erdogan O (2008) The role of inflammation and allergy in acute coronary syndromes. Inflamm Allergy Drug Targets 7:136–144
Rabelo DR, Rocha MO, de Barros MV, Silva JL, Tan TC, Nunes MC (2014) Impaired coronary flow reserve in patients with indeterminate form of Chagas’ disease. Echocardiography 31:67–73
Saraste A, Kyto V, Saraste M, Vuorinen T, Hartiala J, Saukko P (2006) Coronary flow reserve and heart failure in experimental coxsackievirus myocarditis. A transthoracic Doppler echocardiography study. Am J Physiol Heart Circ Physiol 291:H871–H875
Hiss FC, Lascala TF, Maciel BC, Marin-Neto JA, Simoes MV (2009) Changes in myocardial perfusion correlate with deterioration of left ventricular systolic function in chronic Chagas’ cardiomyopathy. JACC Cardiovasc Imaging 2:164–172
Marin-Neto JA, Marzullo P, Marcassa C, Gallo Junior L, Maciel BC, Bellina CR et al (1992) Myocardial perfusion abnormalities in chronic Chagas’ disease as detected by thallium-201 scintigraphy. Am J Cardiol 69:780–784
Simoes MV, Pintya AO, Bromberg-Marin G, Sarabanda AV, Antloga CM, Pazin-Filho A et al (2000) Relation of regional sympathetic denervation and myocardial perfusion disturbance to wall motion impairment in Chagas’ cardiomyopathy. Am J Cardiol 86:975–981
Rossi MA, Tanowitz HB, Malvestio LM, Celes MR, Campos EC, Blefari V, Prado CM (2010) Coronary microvascular disease in chronic Chagas cardiomyopathy including an overview on history, pathology, and other proposed pathogenic mechanisms. PLoS Negl Trop Dis 4:e674
Marin-Neto JA, Simoes MV, Rassi Junior A (2013) Pathogenesis of chronic Chagas cardiomyopathy: the role of coronary microvascular derangements. Rev Soc Bras Med Trop 46:536–541
Zheng XZ, Wu J, Zheng Q, Zha WZ (2016) Coronary sinus flow is reduced and recovered with time in viral myocarditis mimicking acute coronary syndrome: a transthoracic Doppler echocardiographic study. J Ultrasound Med 35:63–69
Vallbracht KB, Schwimmbeck PL, Kuhl U, Rauch U, Seeberg B, Schultheiss HP (2005) Differential aspects of endothelial function of the coronary microcirculation considering myocardial virus persistence, endothelial activation, and myocardial leukocyte infiltrates. Circulation 111:1784–1791
Lee JK, Zaidi SH, Liu P, Dawood F, Cheah AY, Wen WH et al (1998) A serine elastase inhibitor reduces inflammation and fibrosis and preserves cardiac function after experimentally-induced murine myocarditis. Nat Med 4:1383–1391
Tanowitz HB, Kaul DK, Chen B, Morris SA, Factor SM, Weiss LM, Wittner M (1996) Compromised microcirculation in acute murine Trypanosoma cruzi infection. J Parasitol 82:124–130
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 252 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Woudstra, L., Juffermans, L.J.M., van Rossum, A.C. et al. Infectious myocarditis: the role of the cardiac vasculature. Heart Fail Rev 23, 583–595 (2018). https://doi.org/10.1007/s10741-018-9688-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-018-9688-x