Abstract
Sustained hypertension promotes structural, functional and metabolic remodeling of cardiomyocyte mitochondria. As long-lived, postmitotic cells, cardiomyocytes turn over mitochondria continuously to compensate for changes in energy demands and to remove damaged organelles. This process involves fusion and fission of existing mitochondria to generate new organelles and separate old ones for degradation via autophagy. Autophagy is a lysosome-dependent proteolytic pathway capable of processing cellular components, including organelles and protein aggregates. Autophagy can be either nonselective or selective and contributes to remodeling of the myocardium under stress. Fission of mitochondria, loss of membrane potential, and ubiquitination are emerging as critical steps that direct selective autophagic degradation of mitochondria. This review discusses the molecular mechanisms controlling mitochondrial dynamics, including fission, fusion, transport, and degradation. Furthermore, it examines recent studies revealing the importance of these processes in normal and diseased heart.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kearney PM, Whelton M, Reynolds K, et al.: Global burden of hypertension: analysis of worldwide data. Lancet 2005, 365:217–223.
Ingwall JS: Energy metabolism in heart failure and remodelling. Cardiovasc Res 2009, 81:412–419.
• Hom J, Sheu SS: Morphological dynamics of mitochondria--a special emphasis on cardiac muscle cells. J Mol Cell Cardiol 2009, 46:811–820. This is an excellent review of mitochondrial dynamics in cardiomyocytes.
McBride HM, Neuspiel M, Wasiak S: Mitochondria: more than just a powerhouse. Curr Biol 2006, 16:R551–R560.
Scarpulla RC: Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev 2008, 88:611–638.
Russell LK, Mansfield CM, Lehman JJ, et al.: Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage–dependent manner. Circ Res 2004, 94:525–533.
Tolkovsky AM: Mitophagy. Biochim Biophys Acta 2009, 1793:1508–1515.
Kim PK, Hailey DW, Mullen RT, Lippincott-Schwartz J: Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc Natl Acad Sci U S A 2008, 105:20567–20574.
• Taneike M, Yamaguchi O, Nakai A, et al.: Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy 2010 July 1 (Epub ahead of print). This paper demonstrates the importance of autophagy for the maintenance of normal heart function.
Nishida K, Kyoi S, Yamaguchi O, et al.: The role of autophagy in the heart. Cell Death Differ 2009, 16:31–38.
Zhu H, Tannous P, Johnstone JL, et al.: Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest 2007, 117:1782–1793.
Nakai A, Yamaguchi O, Takeda T, et al: The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 2007, 13:619–624.
Rothermel BA, Hill JA: Autophagy in load-induced heart disease. Circ Res 2008, 103:1363–1369.
Skulachev VP: Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem Sci 2001, 26:23–29.
Szabadkai G, Simoni AM, Chami M, et al.: Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2 + -mediated apoptosis. Mol Cell 2004, 16:59–68.
Smirnova E, Griparic L, Shurland DL, van der Bliek AM: Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 2001, 12:2245–2256.
Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM: A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol 1998, 143:351–358.
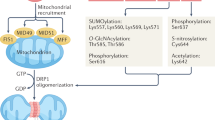
Santel A, Frank S: Shaping mitochondria: The complex posttranslational regulation of the mitochondrial fission protein DRP1. IUBMB Life 2008, 60:448–455.
Cribbs JT, Strack S: Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 2007, 8:939–944.
Han XJ, Lu YF, Li SA, et al.: CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J Cell Biol 2008, 182:573-585.
Eura Y, Ishihara N, Yokota S, Mihara K: Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J Biochem 2003, 134:333–344.
• de Brito OM, Scorrano L: Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 2008, 456:605–610. These studies connect the mitochondrial fusion machinery with Ca 2+ transport between the ER and mitochondria.
Olichon A, Emorine LJ, Descoins E, et al: The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett 2002, 523:171–176.
Song Z, Chen H, Fiket M, et al.: OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol 2007, 178:749–755.
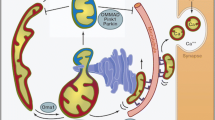
•• Twig G, Elorza A, Molina AJ, et al: Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 2008, 27:433–446. This elegant series of experiments demonstrates that both loss of mitochondrial membrane potential and cleavage of OPA1 help determine whether a daughter mitochondria is degraded by autophagy or fuses to rejoin the mitochondrial network.
•• Narendra D, Tanaka A, Suen DF, Youle RJ: Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 2008, 183:795–803. This paper is the first to demonstrate a functional connection between the Parkinson’s susceptibility gene Parkin and mitophagy. Many subsequent studies have confirmed and expanded these findings.
Matsuda N, Sato S, Shiba K, et al: PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 2010, 189:211–221.
Geisler S, Holmstrom KM, Skujat D, et al.: PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 2010, 12:119–131.
Ziviani E, Whitworth AJ: How could Parkin-mediated ubiquitination of mitofusin promote mitophagy? Autophagy 2010, 6:660–662.
Reis K, Fransson A, Aspenström P: The Miro GTPases: at the heart of the mitochondrial transport machinery. FEBS Lett 2009, 583:1391–1398.
Knott AB, Bossy-Wetzel E: Impairing the mitochondrial fission and fusion balance: a new mechanism of neurodegeneration. Ann N Y Acad Sci 2008, 1147:283–292.
Yu T, Jhun BS, Yoon Y: High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxid Redox Signal 2010 Aug 23 (Epub ahead of print).
Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC: Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol 2003, 160:1115–1127.
Dedkova EN, Blatter LA: Mitochondrial Ca2+ and the heart. Cell Calcium 2008, 44:77–91.
Zorzano A, Liesa M, Sebastian D, et al.: Mitochondrial fusion proteins: dual regulators of morphology and metabolism. Semin Cell Dev Biol 2010, 21:566–574.
Yu T, Sheu SS, Robotham JL, Yoon Y: Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res 2008, 79:341–351.
Marambio P, Toro B, Sanhueza C, et al: Glucose deprivation causes oxidative stress and stimulates aggresome formation and autophagy in cultured cardiac myocytes. Biochim Biophys Acta 2010, 1802:509–518.
Autret A, Martin SJ: Bcl-2 family proteins and mitochondrial fission/fusion dynamics. Cell Mol Life Sci 2010, 67:1599–1606.
Zhang J, Ney PA: Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ 2009, 16:939–946.
Daido S, Kanzawa T, Yamamoto A, et al.: Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res 2004, 64:4286–4293.
Parra V, Eisner V, Chiong M, et al: Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res 2008, 77:387–397.
• Dorn GW 2nd: Mitochondrial pruning by Nix and BNip3: an essential function for cardiac-expressed death factors. J Cardiovasc Transl Res 2010, 3:374–383. This manuscript provides primary data demonstrating the need for BNip3 and Nix to maintain normal cardiac function, as well as an excellent review of the mechanisms through which these proteins may act.
Leary SC, Michaud D, Lyons CN, et al.: Bioenergetic remodeling of heart during treatment of spontaneously hypertensive rats with enalapril. Am J Physiol 2002, 283:H540–H548.
de Cavanagh EM, Ferder M, Inserra F, Ferder L: Angiotensin II, mitochondria, cytoskeletal, and extracellular matrix connections: an integrating viewpoint. Am J Physiol 2009, 296:H550–H558.
Mikusova A, Kralova E, Tylkova L, et al.: Myocardial remodelling induced by repeated low doses of isoproterenol. Can J Physiol Pharmacol 2009, 87:641–651.
Gupta A, Gupta S, Young D, et al.: Impairment of ultrastructure and cytoskeleton during progression of cardiac hypertrophy to heart failure. Lab Invest 2010, 90:520–530.
Lukyanenko V, Chikando A, Lederer WJ: Mitochondria in cardiomyocyte Ca2+ signaling. Int J Biochem Cell Biol 2009, 41:1957–1971.
• Chen L, Gong Q, Stice JP, Knowlton AA: Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res 2009, 84:91–99. These studies demonstrate prominent changes in OPA1 levels in both failing human hearts and rodent models of heart failure.
Hom J, Yu T, Yoon Y, et al.: Regulation of mitochondrial fission by intracellular Ca(2+) in rat ventricular myocytes. Biochim Biophys Acta 2010, 1797:913–921.
•• Ong SB, Subrayan S, Lim SY, et al.: Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 2010, 121:2012–2022. This is the first study to demonstrate that pharmacologic inhibition of mitochondrial fission can protect the heart in an in vivo rodent model of ischemia/reperfusion.
Acknowledgments
This research was funded in part by the National Institutes of Health (to J.A.H. and B.A.R.), the American Heart Association (to M.I., J.A.H., and B.A.R.), the American Heart Association-Jon Holden DeHaan Foundation (to J.A.H.), FONDECYT 1080436 (to S.L.), and FONDAP 1501006 (to S.L).
We would like to thank Dr. Randy McMillan and Dr. Thomas Gillette for critical reading of this manuscript.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iglewski, M., Hill, J.A., Lavandero, S. et al. Mitochondrial Fission and Autophagy in the Normal and Diseased Heart. Curr Hypertens Rep 12, 418–425 (2010). https://doi.org/10.1007/s11906-010-0147-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-010-0147-x