Abstract
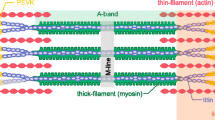
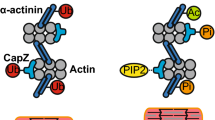
Myosin heads interacting with actin filaments, a process fueled by MgATP and regulated by calcium, powers the pump-like action of the human heart. Hydrolysis of MgATP, the competition between MgATP, its products of hydrolysis, and actin for binding to myosin, and the sequence of shifting affinities in that competition, constitute the central mechanism of muscular contraction. The force, work, and power produced during the cardiac cycle stems from an isomerization of the myosin head that is closely associated with strong binding of myosin to actin and release of phosphate. While fluctuations of intracellular [Ca2+] bound to troponin and related shifts in tropomyosin on the thin filaments regulate the number of crossbridges on a beat-to-beat basis, the oscillatory work produced is augmented by a delayed force response to stretch that develops during diastole. This stretch-activated myogenic response is facilitated by specialized myofilament structures, including actin-binding portions of the myosin essential light chain and myosin binding protein C, which are thought to guide and orient the myosin head or enhance thin filament activation. Phosphorylation of the myosin regulatory light chain, myosin binding protein C, and troponin T also assist in this regard. Animal models show isoform shifts in myosin and other myofibrillar proteins have major effects on power output, but isoform shifts in human myocardium are modest at best and are therefore likely to play only a minor role in modulating crossbridge kinetics compared to disease-related post-translational modifications of the contractile proteins and to changes in their chemical environment.
Similar content being viewed by others
References
Schaub M, Hefti M, Zuellig R, Morano I. Modulation of contractility in human cardiac hypertrophy by myosin essential light chain isoforms. Cardiovasc Res 1998;37:381–404.
Alpert N, Mulieri L, Hasenfuss G. Myocardial chemo-mechanical energy transduction. In: Fozzard HA, et al., eds. The Heart and Cardiovascular System. New York: Raven Press; 1992:111–128.
Alpert NR, Mulieri LA, Warshaw D. The failing human heart. Cardiovasc Res 2002;54:1–10.
Gibbs CL, Chapman JB. Cardiac heat production. Annu Rev Physiol 1979;41:507–519.
Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: A molecular motor. Science 1993;261:50–58.
Sellers JR. Myosins. Second ed. Oxford University Press; 1999.
Sheterline P, Clayton J, Sparrow J. Actin. Oxford University Press; 1998.
Carl SL, Felix K, Caswell AH, Brandt NR, Ball WJ, Jr., Vaghy PL, Meissner G, Ferguson DG. Immunolocalization of sarcolemmal dihydropyridine receptor and sarcoplasmic reticular triadin and ryanodine receptor in rabbit ventricle and atrium. J Cell Biol 1995;129:673–682.
Moolman-Smook J, Flashman E, de Lange W, Li Z, Corfield V, Redwood C, Watkins H. Identification of novel interactions between domains of myosin binding protein-C that are modulated by hypertrophic cardiomyopathy missense mutations. Circ Res 2002;91:704–711.
Alpert NR, Brosseau C, Federico A, Krenz M, Robbins J, Warshaw DM. Molecular mechanics of mouse cardiac myosin isoforms. Am J Physiol Heart Circ Physiol 2002;283:H1446–H1454.
Miyata S, Minobe W, Bristow MR, Leinwand LA. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res 2000;86:386–390.
Reiser PJ, Portman MA, Ning XH, Schomisch MC. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am J Physiol Heart Circ Physiol 2001;280:H1814–H1820.
Fewell JG, Hewett TE, Sanbe A, Klevitsky R, Hayes E, Warshaw D, Maughan D, Robbins J. Functional significance of cardiac myosin essential light chain isoform switching in transgenic mice. J Clin Invest 1998;101:2630–2639.
Morano M, Zacharzowski U, Maier M, Lange PE, Alexi-Meskishvili V, Haase H. Regulation of human heart contractility by essential myosin light chain isoforms. J Clin Invest 1996;98:467–473.
Geeves MA, Lehrer SS. Dynamics of the muscle thin filament regulatory switch: The size of the cooperative unit. Biophys J 1994;67:273–282.
Zhao Y, Kawai M. Kinetic and thermodynamic studies of the cross-bridge cycle in rabbit psoas muscle fibers. Biophys J 1994;67:1655–1668.
Maughan D, Swank D. Insect flight muscle chemomechanics. In: Vigoreaux J, ed. Nature's Versatile Engine: Insect Flight Muscle Inside and Out. Georgetown, TX: Landes Bioscience; 2005:252–270.
Zhao Y, Kawai M. The effect of lattice spacing change on cross-bridge kinetics in chemically skinned rabbit psoas muscle fibers. II. Elementary steps affected by the spacing change. Biophys J 1993;64:197–210.
Holmes KC, Angert I, Kull FJ, Jahn W, Schroder RR. Electron cryo-microscopy shows how strong binding of myosin to actin releases nucleotide. Nature 2003;425:423–427.
Kawai M, Brandt PW. Sinusoidal analysis: A high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscle of rabbit, frog and crayfish. J Mus Res Cell Motil 1980;1:279–303.
Kawai M, Saeki Y, Zhao Y. Crossbridge scheme and the kinetic constants of elementary steps deduced from chemically skinned papillary and trabecular muscles of the ferret. Circ Res 1993;73:35–50.
Maughan D, Moore J, Vigoreaux J, Blanchard E, Mulieri L. Work production and work absorption in muscle strips from vertebrate cardiac and insect flight muscle fibers. In: Sugi H, Pollack G, eds. Mechanism of Work Production and Work Absorption in Muscle. New York: Plenum Publishing Press; 1998:471–480.
Tyska MJ, Warshaw DM. The myosin power stroke. Cell Motil Cytoskeleton 2002;51:1–15.
Cecchi G, Bagni MA. Myofilament lattice spacing affects tension in striated muscle. News Physiol Sci 1994;9:3–7.
Millman BM. The filament lattice of striated muscle. Physiological Rev 1998;78:359–391.
Cooke R. Actomyosin interaction in striated muscle. Physiol Rev 1997;77:671–697.
Nyland L, Maughan D. Surface morphology and transverse stiffness of Drosophila indirect flight muscle myofibrils measured by atomic force microscopy. Biophys J 2000;78:1490–1497.
Mulieri LA, Barnes W, Leavitt BJ, Ittleman FP, LeWinter MM, Alpert NR, Maughan DW. Alterations of myocardial dynamic stiffness implicating abnormal crossbridge function in human mitral regurgitation heart failure. Circ Res 2002;90:66–72.
Kodama T. Thermodynamic analysis of muscle ATPase mechanisms. Physiol Rev 1985;65:467–551.
Woledge RC, Reilly PJ. Molar enthalpy change in the hydrolysis of phosphocreatine under conditions in muscle cells. Biophys J 1988;54:97–104.
Godt RE, Maughan DW. On the composition of the cytosol of relaxed skeletal muscle of the frog. Am J Physiol (Cell Physiol) 1988;254:C591–C604.
Sweeney HL. The importance of the creatine kinase reaction: The concept of metabolic capacitance. Med Sci Sports Exerc 1994;26:30–36.
Homsher E, Lacktis J, Regnier M. Strain-dependent modulation of phosphate transients in rabbit skeletal muscle fibers. Biophys J 1997;72:1780–1791.
Hinken AC, McDonald KS. Inorganic phosphate speeds loaded shortening in rat skinned cardiac myocytes. Am J Physiol Cell Physiol 2004;287:C500–C507.
Fukagawa NK, Palmer BM, Barnes WD, Leavitt BJ, Ittleman FP, LeWinter MM, Mulieri LA, Maughan DW. Acto-myosin crossbridge kinetics in humans with coronary artery disease: Influence of sex and diabetes mellitus. J Mol Cell Cardiol 2005;(in press).
Hajjar RJ, Gwathmey JK. Cross-bridge dynamics in human ventricular myocardium-regulation of contractility in the failing human heart. Circulation 1992;86:1819–1826.
Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev 2000;80:853–924.
Opitz CA, Kulke M, Leake MC, Neagoe C, Hinssen H, Hajjar RJ, Linke WA. Damped elastic recoil of the titin spring in myofibrils of human myocardium. Proc Natl Acad Sci U S A 2003;100:12688–12693.
Palmer BM, Georgakopoulos D, Janssen PM, Wang Y, Alpert NR, Belardi DF, Harris SP, Moss RL, Burgon PG, Seidman CE, Seidman JG, Maughan DW, Kass DA. Role of cardiac myosin binding protein C in sustaining left ventricular systolic stiffening. Circ Res 2004;94:1249–1255.
Araujo A, Walker JW. Kinetics of tension development in skinned cardiac myocytes measured by photorelease of Ca+. Am J Physiol 1994;267:H1643–H1653.
Araujo A, Walker JW. Phosphate release and force generation in cardiac myocytes investigated with caged phosphate and caged calcium. Biophys J 1996;70:2316–2326.
Stehle R, Kruger M, Scherer P, Brixius K, Schwinger RH, Pfitzer G. Isometric force kinetics upon rapid activation and relaxation of mouse, guinea pig and human heart muscle studied on the subcellular myofibrillar level. Basic Res Cardiol 2002;97 Suppl 1:I127–I135.
Alpert NR, Peterson JN. A molecular biophysical approach to contraction and relaxation. In: Lorell BH, Grossman W, eds. Diastolic Relaxation of the Heart. Boston: Kluwer; 1994:66–71.
Fuchs F, Smith SH. Calcium, cross-bridges, and the Frank-Starling relationship. News Physiol Sci 2001;16:5–10.
Irving TC, Konhilas J, Perry D, Fischetti R, de Tombe PP. Myofilament lattice spacing as function of sarcomere length in isolated rat myocardium. Am J Physiol Heart Circ Physiol 2000;279:H2568–H2573.
Kawai M, Wray JS, Zhao Y. The effect of lattice spacing change on cross-bridge kinetics in chemically skinned rabbit psoas muscle fibers. Biophys J 1993;64:187–196.
Martyn DA, Gordon AM. Length and myofilament spacing-dependent changes in calcium sensititivy of skeletal fibres: Effects of pH and ionic strength. J Mus Res Cell Motil 1988;9:428–445.
Metzger JM, Moss RL. Thin filament regulation of shortening velocity in rat skinned skeletal muscle: Effects of osmotic compression. J Physiol 1988;398:165–175.
Konhilas J, Irving TC, de Tombe PP. Myofilament calcuim sensitivity in skinned rat cardiac trabeculae: Role of interfilament spacing. Circ Res 2002;90:59–65.
Dickinson MH, Hyatt CJ, Lehmann F-O, Moore JR, Reedy MC, Simcox A, Tohtong R, Vigoreaux JO, Yamashita H, Maughan DW. Phosphorylation-dependent power output of transgenic flies: An integrated study. Biophys J 1997;73:3122–3134.
Noland JrTA, Kuo JF. Protein kinase C phosphorylation of cardiac Troponin I and Troponin T inhibits Ca2+-stimulated MgATPase activity in reconstituted actomyosin and isolated myofibrils, and decreases actin-myosin interactions. J Mol Cell Cardiol 1993;25:53–65.
Sweeney HL, Bowman BF, Stull JT. Myosin light chain phosphorylation in vertebrate striated muscle: Regulation and function. Am J Physiol 1993;264:C1085–C1095.
Gautel M, Zuffardi O, Freiburg A, Labeit S. Phosphorylation switches specific for the cardiac isoform of myosin binding protein-C: A modulator of cardiac contraction? The EMBO Journal 1995;14:1952–1960.
Kunst G, Kress KR, Gruen M, Uttenweiler D, Gautel M, Fink RHA. Myosin binding protein C, a phosphorylation-dependent force regulator in muscle that controls the attachment of myosin heads by its interaction with myosin S2. Circ Res 2000;86:51–58.
Venema RC, Kuo JF. Protein kinase C-mediated phosphorylation of troponin I and C-protein in isolated myocardial cells is associated with inhibition of myofibrillar actomyosin MgATPase. J Biol Chem 1993;268(4):2705–2711.
90. Weisberg A, Winegrad S. Alteration of myosin cross bridges by phosphorylation of myosin-binding protein C in cardiac muscle. Proc Natl Acad Sci 1996;93:8999–9003.
Winegrad S, McClellan G, Horowits R, Tucker M, Lin L-E, Weisberg A. Regulation of cardiac contractile proteins by phosphorylation. Federation Proc 1983;42:39–44.
Squire JM, Luther PK, Knupp C. Structural Evidence for the Interaction of C-protein (MyBP-C) with Actin and Sequence Identification of a Possible Actin-binding Domain. J Mol Biol 2003;331:713–724.
Epstein ND, Davis JS. Sensing stretch is fundamental. Cell 2003;112:147–150.
Edman KAP. Mechanical deactivation induced by active shortening in isolated muscle fibres of the frog. J Physiol 1975;246:255–275.
Pringle JWS. The Croonian Lecture, 1977. Stretch activation of muscle; function and mechanism. Proc Roy Soc London B 1978;201:107–130.
Blanchard E, Seidman C, Seidman J, LeWinter M, Maughan D. Ca2+ sensitivity of isometric tension, oscillatory power, and complex stiffness parameters of cardiac muscle from an α -myosin heavy chain R403Q mouse model of human familial hypertrophic cardiomyopathy. Circ Res 1999;84:475–483.
Steiger GJ. Stretch activation and tension transients in cardiac, skeletal and insect flight muscle. In: Tregear RT, ed. Insect Flight Muscle Amsterdam: Elsevior–North Holland Biomedical Press, 1977:221–268.
Maughan D, Moore J, Vigoreaux J, Barnes B, Mulieri LA. Work production and work absorption in muscle strips from vertebrate cardiac and insect flight muscle fibers. Adv Exp Med Biol 1998;453:471–480.
Vemuri R, Lankford EB, Poetter K, Hassanzadeh S, Takeda K, Yu Z, Ferrans VJ, Epstein ND. The stretch-activation response may be critical to the proper functioning of the mammalian heart. Proc Natl Acad Sci USA 1999;96:1048–1053.
Poetter K, Jiang H, Hassanzadeh S, Master SR, Chang A, Dalakas MC, Rayment I, Sellers JR, Fananapazir L, Epstein ND. Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nature Genetics 1996;13:63–69.
von Lewinski D, Stumme B, Fialka F, Luers C, Pieske B. Functional relevance of the stretch-dependent slow force response in failing human myocardium. Circ Res 2004;94:1392–1398.
McDonald KS. Ca2+ dependence of loaded shortening in rat skinned cardiac myocytes and skeletal muscle fibers. J Physiol 2000;525:169–181.
Winegrad S. Cardiac Myosin Binding Protein C. Circ Res 1999;84:1117–1126.
Sweeney HL. Function of the N Terminus of the myosin essential light chain of vertebrate striated muscle. Biophys J 1995;68:112s–119s.
Hofmann PA, Lange JH. Effects of phosphorylation of Troponin I and C protein on isometric tension and velocity of unloaded shortening in skinned single cardiac myocytes from rats. Circ Res 1994;74:718–726.
Bottinelli R. Functional heterogenetiy of mammalian single muscle fibres: Do myosin isoform tell the whole story? Pflueg Arch 2001;443:7–17.
Lowey S, Waller GS, Trybus KM. Function of skeletal muscle myosin heavy and light chain isoforms by an in vitro motility assay. J Biol Chem 1993;268:20414–20418.
Swank DM, Kronert WA, Bernstein SI, Maughan DW. Alternative N-terminal regions of Drosophila myosin heavy chain tune muscle kinetics for optimal power output. Biophys J 2004;87:1805–1814.
Gorza L, Mercadier JJ, Schwartz K, Thornell LE, Sartore S, Schiaffino S. Myosin types in the human heart. An immunofluorescence study of normal and hypertrophied atrial and ventricular myocardium. Circ Res 1984;54:694–702.
Mercadier JJ, Bouveret P, Gorga L, Schiaffino S, Clark WA, Zak R, Swynghedauw B, Schwartz K. Myosin isoenzymes in normal and hypertrophied human ventricular myocardium. Circ Res 1983;53:52–62.
Noguchi T, Camp P, Jr., Alix SL, Gorga JA, Begin KJ, Leavitt BJ, Ittleman FP, Alpert NR, LeWinter MM, VanBuren P. Myosin from failing and non-failing human ventricles exhibit similar contractile properties. J Mol Cell Cardiol 2003;35:91–97.
Morano I, Bletz C, Wojciechowski R, Ruegg JC. Modulation of crossbridge kinetics by myosin isoenzymes in skinned human heart fibers. Circ Res 1991;68:614–618.
Morano I, Arndt H, Gartner C, Ruegg JC. Skinned fibers of human atrium and ventricle: Myosin isoenzymes and contractility. Circ Res 1988;62:632–639.
Yamashita H, Sugiura S, Fujita H, Yasuda S, Nagai R, Saeki Y, Sunagawa K, Sugi H. Myosin light chain isoforms modify force-generating ability of cardiac myosin by changing the kinetics of actin-myosin interaction. Cardiovasc Res 2003;60:580–588.
Hirzel HO, Tuchschmid CR, Schneider J, Krayenbuehl HP, Schaub MC. Relationship between myosin isoenzyme composition, hemodynamics, and myocardial structure in various forms of human cardiac hypertrophy. Circ Res 1985;57:729–740.
Sylven C, Jannson ESABK. Key enzymes of myocardial energy metabolism in patients with valvular heart disease: Relation to left ventricular function. Acta Physiol Scand 1988;132:267–270.
Wallimann T, Schlosser T, Eppenberger HM. Function of M-line-bound creatine kinase as intramyofibrillar ATP regenerator at the receiving end of the phosphorylcreatine shuttle in muscle. J Biol Chem 1984;259:5238–5246.
van der Velden J, Papp Z, Boontje NM, Zaremba R, de Jong JW, Janssen PM, Hasenfuss G, Stienen GJ. The effect of myosin light chain 2 dephosphorylation on Ca2+ -sensitivity of force is enhanced in failing human hearts. Cardiovasc Res 2003;57:505–514.
van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ, Burton PB, Goldmann P, Jaquet K, Stienen GJ. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res 2003;57:37–47.
Brixius K, Savvidou-Zaroti P, Mehlhorn U, Bloch W, Kranias EG, Schwinger RH. Increased Ca2+-sensitivity of myofibrillar tension in heart failure and its functional implication. Basic Res Cardiol 2002;97 (Suppl 1):I111–I117.
Schwinger RH, Bohm M, Koch A, Schmidt U, Morano I, Eissner HJ, Uberfuhr P, Reichart B, Erdmann E. The failing human heart is unable to use the Frank-Starling mechanism. Circ Res 1994;74:959–969.
Anderson PAW, Greig A, Mark TM, Malouf NN, Oakeley AE, Ungerleider RM, Allen PD, Kay BK. Molecular basis of human cardiac troponin T isoforms expressed in the developing, adult and failing heart. Circ Res 1995;76:681–686.
Anderson PAW, Malouf NN, Oakeley AE, Pagani ED, Allen PD. Troponin T isoform expression in humans: A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circ Res 1991;69:1226–1233.
Margossian SS, White HD, Caulfield JB, Norton P, Taylor S, Slayter HS. Light chain 2 profile and activity of human ventricular myosin during dilated cardiomyopathy - Identification of a casual agent for impaired myocardial function. Circulation 1992;85:1720–1733.
Wolff MR, Buck SH, Stoker SW, Greaser ML, Mentzer RM. Myofibrillar calcium sensitivity of isometric tension is increased in human dilated cardiomyopathies: Role of altered beta-adrenergically mediated protein phosphorylation. J Clin Invest 1996;98:167–176.
Schmidt U, Hajjar RJ, Helm PA, Kim CS, Doye AA, Gwathmey JK. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J Mol Cell Cardiol 1998;30:1929–1937.
Davies CH, Davia K, Bennett JG, Pepper JR, Poole-Wilson PA, Harding SE. Reduced contraction and altered frequency response of isolated ventricular myocytes from patients with heart failure. Circulation 1995;92:2540–2549.
Arai M, Matsui H, Periasamy M. Sarcoplasmic reticulum gene expression in cardiac hypertrophy and heart failure. Circ Res 1994;74:555–564.
del Monte F, Harding SE, Schmidt U, Matsui T, Kang ZB, Dec GW, Gwathmey JK, Rosenzweig A, Hajjar RJ. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation 1999;100:2308–2311.
Anderson PAW, Malouf NN, Oakeley AE, Pagani ED, Allen PD. Troponin T isoform expression in the normal and failing human left ventricle: A correlation with myofibrillar ATPase activity. Basic Res Cardiol 1992;87:117–127.
Torrealba JR, Lozano E, Griffin M, Stoker S, McDonald K, Greaser M, Wolff MR. Maximal ATPase activity and calcium sensitivity of reconstituted myofilaments are unaltered by the fetal troponin T re-expressed during human heart failure. J Mol Cell Cardiol 2002;34:797–805.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maughan, D.W. Kinetics and Energetics of The Crossbridge Cycle. Heart Fail Rev 10, 175–185 (2005). https://doi.org/10.1007/s10741-005-5248-2
Issue Date:
DOI: https://doi.org/10.1007/s10741-005-5248-2