Abstract
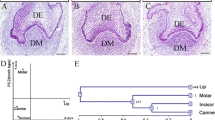
We previously performed cDNA subtraction between the mouse mandibles on embryonic day 10.5 (E10.5) in the pre-initiation stage of the odontogenesis and E12.0 in the late initiation stage to identify genes expressed at its beginning. Adenosine triphosphate synthase subunit a (Atpase6) is one of the highly expressed genes in the E12.0 mandible including tooth germs. In situ hybridization was conducted using the mouse mandibular first molar from E10.5 to E18.0 to determine the precise expression patterns of Atpase6 mRNA in the developing tooth germ. Atpase6 mRNA was strongly expressed in the presumptive dental epithelium and the underlying mesenchyme at E10.5, and in the thickened dental epithelium at E12.0 and E13.0. Strong in situ signals were observed in the epithelium at E14.0, and in the enamel organ excluded the area of the primary enamel knot at E15.0. Atpase6 was strongly expressed in the inner enamel epithelium, the adjacent stratum intermedium, and the outer enamel epithelium in the cervical loops from E16.0 to E18.0. In addition, strong Atpase6 signals were coincidently demonstrated in various developing cranio-facial organs. These results suggest that Atpase6 participates in the high energy-utilizing functions of the cells related to the initiation and the development of the tooth germ as well as those of the other cranio-facial organs.




Similar content being viewed by others
References
Abrahams JP, Leslie AG, Lutter R, Walker JE (1994) Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature 370:621–628. doi:10.1038/370621a0
Akhter M, Kobayashi I, Kiyoshima T, Matsuo K, Yamaza H, Wada H, Honda JY, Ming X, Sakai H (2005) Possible functional involvement of thymosin beta 4 in developing tooth germ of mouse lower first molar. Histochem Cell Biol 124:207–213. doi:10.1007/s00418-005-0040-x
Akhter M, Kobayashi I, Kiyoshima T, Nagata K, Wada H, Ookuma Y, Fujiwara H, Honda J Y, Sakai H (2010) In situ expression of 15 kDa interferon alpha responsive gene in the developing tooth germ of the mouse lower first molar. J Mol Histol. doi: 10.1007/s10735-010-9277-3
Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG (1981) Sequence and organization of the human mitochondrial genome. Nature 290:457–465
Bibb MJ, Van Etten RA, Wright CT, Walberg MW, Clayton DA (1981) Sequence and gene organization of mouse mitochondrial DNA. Cell 26:167–180
Boyer PD (1993) The binding change mechanism for ATP synthase–some probabilities and possibilities. Biochim Biophys Acta 1140:215–250
Boyer PD (1997) The ATP synthase–a splendid molecular machine. Annu Rev Biochem 66:717–749. doi:10.1146/annurev.biochem.66.1.717
Burrell HE, Wlodarski B, Foster BJ, Buckley KA, Sharpe GR, Quayle JM, Simpson AW, Gallagher JA (2005) Human keratinocytes release ATP and utilize three mechanisms for nucleotide interconversion at the cell surface. J Biol Chem 280:29667–29676. doi:10.1074/jbc.M505381200
D’Souza R (2007) Development of the tooth and its supporting tissues In: Nanci A (ed) Ten Cate’s oral histology: development, structure, and function, 7th edn. Mosby Elsevier, St. Louis, pp 79–107
Das B, Mondragon MO, Sadeghian M, Hatcher VB, Norin AJ (1994) A novel ligand in lymphocyte-mediated cytotoxicity: expression of the beta subunit of H + transporting ATP synthase on the surface of tumor cell lines. J Exp Med 180:273–281
Decker JD (1963) A light and electron microscope study of the rat molar enamel organ. Arch Oral Biol 8:301–310
Dixon CJ, Bowler WB, Littlewood-Evans A, Dillon JP, Bilbe G, Sharpe GR, Gallagher JA (1999) Regulation of epidermal homeostasis through P2Y2 receptors. Br J Pharmacol 127:1680–1686. doi:10.1038/sj.bjp.0702653
Egawa I (1970) An electron microscope study of the human enamel organ (translated from Japanese title). Shikwa Gakuho 70:803–836
Fang P, Wang X, Zhang L, Yuan G, Chen Z, Zhang Q (2010) Immunohistochemical localization of LIM mineralization protein 1 during mouse molar development. J Mol Histol 41:199–203. doi:10.1007/s10735-010-9279-1
Fincham AG, Moradian-Oldak J, Simmer JP (1999) The structural biology of the developing dental enamel matrix. J Struct Biol 126:270–299. doi:10.1006/jsbi.1999.4130
Fischlschweiger W, Provenza DV, Sisca RF (1967) Reorganization of the peripheral layers of the human enamel organ during the bell stage–an electron microscopic study. J Baltimore Coll Dent Surg 22:28–55
Greig AV, Linge C, Terenghi G, McGrouther DA, Burnstock G (2003) Purinergic receptors are part of a functional signaling system for proliferation and differentiation of human epidermal keratinocytes. J Invest Dermatol 120:1007–1015
Groschel-Stewart U, Bardini M, Robson T, Burnstock G (1999) Localisation of P2X5 and P2X7 receptors by immunohistochemistry in rat stratified squamous epithelia. Cell Tissue Res 296:599–605
Honda JY, Kobayashi I, Kiyoshima T, Yamaza H, Xie M, Takahashi K, Enoki N, Nagata K, Nakashima A, Sakai H (2008) Glycolytic enzyme Pgk1 is strongly expressed in the developing tooth germ of the mouse lower first molar. Histol Histopathol 23:423–432
Ikemoto A, Bole DG, Ueda T (2003) Glycolysis and glutamate accumulation into synaptic vesicles. Role of glyceraldehyde phosphate dehydrogenase and 3-phosphoglycerate kinase. J Biol Chem 278:5929–5940. doi:10.1074/jbc.M211617200
Jernvall J, Kettunen P, Karavanova I, Martin LB, Thesleff I (1994) Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int J Dev Biol 38:463–469
Kobayashi I, Kiyoshima T, Wada H, Matsuo K, Nonaka K, Honda JY, Koyano K, Sakai H (2006) Type II/III Runx2/Cbfa1 is required for tooth germ development. Bone 38:836–844. doi:10.1016/j.bone.2005.10.026
Ohshima H, Wartiovaara J, Thesleff I (1999) Developmental regulation and ultrastructure of glycogen deposits during murine tooth morphogenesis. Cell Tissue Res 297:271–281
Olson M (1997) Bioenergetics and oxidative metabolism. In: Devlin T (ed) Textbook of biochemistry with clinical correlations. edn. Wiley-Liss, New York, pp 217–263
Shigemura N, Kiyoshima T, Kobayashi I, Matsuo K, Yamaza H, Akamine A, Sakai H (1999) The distribution of BrdU- and TUNEL-positive cells during odontogenesis in mouse lower first molars. Histochem J 31:367–377
Sisca RF, Provenza DV, Fischlschweiger W (1967) Ultrastructural characteristics of the human enamel organ in an early stage of development. J Baltimore Coll Dent Surg 22:8–27
Skou JC (1974) Effect of ATP on the intermediary steps of the reaction of the (Na + plus K +)-dependent enzyme system. 3. Effect on the p-nitrophenylphosphatase activity of the system. Biochim Biophys Acta 339:258–273
Smith CE (1998) Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med 9:128–161
Thesleff I (2006) The genetic basis of tooth development and dental defects. Am J Med Genet A 140:2530–2535. doi:10.1002/ajmg.a.31360
Thesleff I, Vaahtokari A, Kettunen P, Aberg T (1995) Epithelial-mesenchymal signaling during tooth development. Connect Tissue Res 32:9–15
Wada H, Kobayashi I, Yamaza H, Matsuo K, Kiyoshima T, Akhtar M, Sakai T, Koyano K, Sakai H (2002) In situ expression of heat shock proteins, Hsc73, Hsj2 and Hsp86 in the developing tooth germ of mouse lower first molar. Histochem J 34:105–109
Xie M, Kobayashi I, Kiyoshima T, Yamaza H, Honda JY, Takahashi K, Enoki N, Akamine A, Sakai H (2007) Functional implication of nucleolin in the mouse first molar development. J Biol Chem 282:23275–23283. doi:10.1074/jbc.M610779200
Xie M, Kobayashi I, Kiyoshima T, Nagata K, Ookuma Y, Fujiwara H, Sakai H (2009) In situ expression of ribosomal protein L21 in developing tooth germ of the mouse lower first molar. J Mol Histol. doi: 10.1007/s10735-009-9249-7
Yamaza H, Matsuo K, Kiyoshima T, Shigemura N, Kobayashi I, Wada H, Akamime A, Sakai H (2001a) Detection of differentially expressed genes in the early developmental stage of the mouse mandible. Int J Dev Biol 45:675–680
Yamaza H, Matsuo K, Kobayashi I, Wada H, Kiyoshima T, Akhtar M, Ishibashi Y, Sakai T, Akamine A, Sakai H (2001b) Expression of Set-alpha during morphogenesis of mouse lower first molar. Histochem J 33:437–441
Yoshida M, Muneyuki E, Hisabori T (2001) ATP synthase–a marvellous rotary engine of the cell. Nat Rev Mol Cell Biol 2:669–677. doi:10.1038/35089509
Zhang X, Gao F, Yu LL, Peng Y, Liu HH, Liu JY, Yin M, Ni J (2008) Dual functions of a monoclonal antibody against cell surface F1F0 ATP synthase on both HUVEC and tumor cells. Acta Pharmacol Sin 29:942–950. doi:10.1111/j.1745-7254.2008.00830.x
Zhao Y, Zhang W, Kho Y (2004) Proteomic analysis of integral plasma membrane proteins. Anal Chem 76:1817–1823. doi:10.1021/ac0354037
Acknowledgments
The authors wish to acknowledge support by a Grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (20390466 to H.S. and 21592331 to I.K.). The authors thank Dr. K. Matsuo for his discussion.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jun-ya Honda, Ieyoshi Kobayashi and Tamotsu Kiyoshima contributed equally to this work.
Rights and permissions
About this article
Cite this article
Honda, Jy., Kobayashi, I., Kiyoshima, T. et al. In situ expression of the mitochondrial ATPase6 gene in the developing tooth germ of the mouse lower first molar. J Mol Hist 42, 83–90 (2011). https://doi.org/10.1007/s10735-010-9309-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-010-9309-z