Abstract
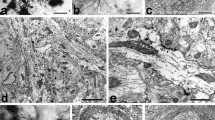
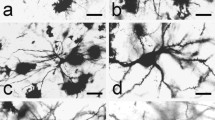
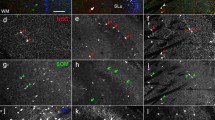
Nitric oxide is a unique neurotransmitter, which participates in many physiological and pathological processes in the organism. Nevertheless there are little data about the neuronal Nitric Oxide Synthase immunoreactive (nNOS-ir) neurons and fibers in the dorsal claustrum (DC) of a cat. In this respect the aims of this study were: (1) to demonstrate nNOS-ir in the neurons and fibers of the DC; (2) to describe their light microscopic morphology and distribution; (3) to investigate and analyze the ultrastructure of the nNOS-ir neurons, fibers and synaptic terminals; (4) to verify whether the nNOS-ir neurons consist a specific subpopulation of claustral neurons; (5) to verify whether the nNOS-ir neurons have a specific pattern of organization throughout the DC. For demonstration of the nNOS-ir the Avidin-Biotin-Peroxidase Complex method was applied. Immunopositive for nNOS neurons and fibers were present in all parts of DC. On the light microscope level nNOS-ir neurons were different in shape and size. According to the latter they were divided into three groups—small (with diameter under 15 μm), medium-sized (with diameter from 16 to 20 μm) and large (with diameter over 21 μm). Some of nNOS-ir neurons were lightly-stained while others were darkly-stained. On the electron microscope level the immunoproduct was observed in neurons, dendrites and terminal boutons. Different types of nNOS-ir neurons differ according to their ultrastructural features. Three types of nNOS-ir synaptic boutons were found. As a conclusion we hope that the present study will contribute to a better understanding of the functioning of the DC in cat and that some of the data presented could be extrapolated to other mammals, including human.




Similar content being viewed by others
References
Aoki C, Rhee J, Lubin M et al (1997) NMDA-R1 subunit of the cerebral cortex co-localizes with neuronal nitric oxide synthase at pre- and postsynaptic sites and in spines. Brain Res 750:25–40. doi:10.1016/S0006-8993(96)01147-X
Ashwell KWS, Hardman C, Paxinos G (2004) The claustrum is not missing from all monotreme brains. Brain Behav Evol 64:223–241. doi:10.1159/000080243
Berke JJ (1960) The claustrum, the external capsule and the extreme capsule of Macaca mulatta. Neurology 115:297–321
Berlucchi C (1927) Richerche di fine anatomia sul claustrum e sull’ insula dell gate. Riv Sperim Freniatria 51:125–157 (Microscopic anatomy of the claustrum and insula of the cat)
Bishop A, Anderson JE (2005) NO signaling in the CNS: from the physiological to the pathological. Toxicology 208:193–205. doi:10.1016/j.tox.2004.11.034
Blumcke I, Hof PR, Morrison JH et al (1991) Parvalbumin in the monkey striate cortex: a quantitative immunoelectron microscopy study. Brain Res 554:237–243. doi:10.1016/0006-8993(91)90195-2
Braak H, Braak E (1982) Neuronal types in the claustrum of man. Anat Embryol (Berl) 163:473–488. doi:10.1007/BF00305558
Brand S (1981) A serial section Golgi analysis of the primate claustrum. Anat Embryol (Berl) 162:447–460. doi:10.1007/BF00301872
Brockhaus H (1940) Cytoarchitectural and myeloarchitectural study of claustral cortex and claustrum in man. J Psychol Neurol 49:249–348
Carey RG, Neal TL (1985) The rat claustrum; afferent and efferent connections with visual cortex. Brain Res 329:185–193. doi:10.1016/0006-8993(85)90524-4
Carey RG, Bear MF, Diamond IT (1980) The laminar organization of the reciprocal projections between the claustrum and the striate cortex in the tree shrew, Tupaia glis. Brain Res 184:193–198. doi:10.1016/0006-8993(80)90597-1
Czeiger D, White EL (1997) Comparison of the distribution of parvalbumin-immunoreactive and other synapses onto the somata of callosal projection neurons in mouse visual and somatosensory cortex. J Comp Neurol 379:198–210. doi :10.1002/(SICI)1096-9861(19970310)379:2<198::AID-CNE3>3.0.CO;2-Z
Dahrmann G, Gossrau R (1996) Nitric oxide synthase (NOS) I immunoreactivity and NOS-associated-NADPH diaphorase (NOSaNADPHd) histochemistry in mouse skeletal muscles during postnatal developmnt. Ann Anat 178:229–230
Dalkara T, Moskowitz MA (1994) The complex role of nitric oxide in the pathophysiology of focal cerebral ischemia. Brain Pathol 4:49–57
Dawson DA (1994) Nitric oxide and focal cerebral ischemia: multiplicity of actions and diverse outcome. Cerebrovasc Brain Metab Rev 6:299–324
Dawson VL, Dawson TM, London ED et al (1991a) Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA 15:6368–6371. doi:10.1073/pnas.88.14.6368
Dawson TM, Bredt DS, Fotuhi M et al (1991b) Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissue. Proc Natl Acad Sci USA 88:7797–7801
Dawson VL, Dawson TM, Bartley DA et al (1993) Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J Neurosci 13:2651–2661
De Vries E (1910) Bemerkungen zur ontogenie und vergleichenden anatomie des ckaustrums. Folia Neurobiol 4:481–513
Dinopoulos A, Papadopoulos GC, Michaloudi H et al (1992) Claustrum in the hedgehog Erinaceus europaeus brain: cytoarchitecture and connections with cortical and subcortical structures. J Comp Neurol 316:187–205. doi:10.1002/cne.903160205
Dohrn CS, Beitz AJ (1994) NMDA receptor mRNA expression in NOS-containing neurons in the spinal trigeminal nucleus of the rat. Neurosci Lett 175:28–32. doi:10.1016/0304-3940(94)91070-7
Druga R (1966a) The claustrum of the cat (Felis domestica). Folia Morphol (Praha) 14:7–16
Druga R (1966b) Cortico-claustral connections. I. Fronto-claustral connections. Folia Morphol (Praha) 14:391–399
Druga R (1968) Cortico-claustral connections. II. Connections from the parietal, temporal and occipital cortex to the claustrum. Folia Morphol (Praha) 16:142–149
Druga R (1971) Projection of prepyriform cortex into claustrum. Folia Morphol (Praha) 19:405–410
Druga R (1974) The claustrum and the transitional neopaleocortical area of the hedgehog (Erinacea Europaeus). Anat Anz Jena 135:442–454
Druga R (1975) Claustrum (Struktura, Ontogenese a Spoje). Doctoral dissertation. Charles University, Praha, 193 p
Druga R, Chen S, Bentivoglio M (1993) Parvalbumin and calbindin in the rat claustrum; an immunocytochemical study combined with retrograde tracing from frontoparietal cortex. J Chem Neuroanat 6:399–406. doi:10.1016/0891-0618(93)90014-U
Edelstein LR, Denaro FJ (2004) The claustrum: a historical review of its anatomy, physiology, cytochemistry and functional significance. Cell Mol Biol 50:675–702
Edelstein LR (1986) The anatomy of the claustrum: a light- and electron-microscopic analysis in rat and monkey incorporating the technique of HRP cytochemistry, Thesis, State University of New York at Stony Brook, New York, 279 pp. (incl. 66 figures)
Filimonoff IN (1966) The claustrum: its origin and development. J Hirnforsch 8:503–528
Gracy KN, Pickel VM (1997) Ultrastructural localization and comparative distinction of nitric oxide sybthase and N-methyl-D-aspartate receptors in the shell of the rat nucleus accumbens. Brain Res 747:259–272. doi:10.1016/S0006-8993(96)01249-8
Guirado S, Real MA, Olmos JL, Davila JC et al (2003) Distinct types of nitric oxide-producing neurons in the developing and adult mouse claustrum. J Comp Neurol 465:431–444. doi:10.1002/cne.10835
Hinova-Palova D (1981) Identification of degenerated boutons in claustrum dorsale after lesion of visual cortex. C R Acad bulg Sci 34:449–452
Hinova-Palova D (1986) Light-microscopic and ultrastructural organization of the claustrum in the cat. Afferent and efferent connections. Thesis, Medical Academy, Sofia
Hinova-Palova DV, Paloff AM (1982) Corticoclaustral connections. An electron-microscopic study. Verh Anat Ges 76:503–504
Hinova-Palova D, Paloff A (1984) Identification of degenerated synaptic boutons in the claustrum of cat after lesion of the parietal cortex. Contemp Probl Neuromorphol Sofia 13–14:154–160
Hinova-Palova DV, Paloff AM, Usunoff KG (1980a) Identification of three types of degenerated boutons in claustrum dorsale of the cat after lesion of the temporal cortex. C R Acad Bulg Sci 33:125–128
Hinova-Palova DV, Paloff AM, Usunoff KG (1980b) Identification of three types of degenerated boutons in claustrum dorsale of the cat after lesion of the frontal cortex. C R Acad Bulg Sci 33:129–132
Hinova-Palova D, Paloff A, Usunoff K, Dimova R, Wossifov T, Ivanov D (1988) Reciprocal connections between the claustrum and the auditory cortical field in the cat. An experimental study using light and electron microscopic anterograde degeneration methods and the horseradish peroxidase retrograde axonal transport. J Hirnforsch 29:255–278
Hinova-Palova DV, Paloff AM, Christova T et al (1997) Topographical distribution of NADPH-diaphorase-positive neurons in the cat’s claustrum. Eur J Morphol 35:105–116. doi:10.1076/ejom.35.2.105.13068
Hinova-Palova D, Papantchev V, Hristov S, Paloff A, Ovtscharoff W (2005) Light and electron microscopical demonstration of nNOS immunoreactivity in cat’s claustrum. On congress CD of XVII Congress of Anatomy with international participation, 10–12 June, Hissar, Bulgaria
Hinova-Palova D, Edelstein L, Paloff A et al (2007) Parvalbumin in the cat claustrum: ultrastructure, distribution and functional implications. Acta Histochem 109:61–77. doi:10.1016/j.acthis.2006.09.006
Holstein GR, Friedrich VL, Martinelli GP (2001) Monoclonal L-citrulline immunostaining reveals nitric oxide-producing vestibular neurons. Ann N Y Acad Sci 942:65–78
Hope BT, Michael GJ, Knigge KM et al (1991) Vincent SR. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci USA 88:2811–2814. doi:10.1073/pnas.88.7.2811
Iadecola C (1997) Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci 20:132–139. doi:10.1016/S0166-2236(96)10074-6
Kowianski P, Morys J, Karwacki Z (1998) The cortico-related zones of the rabbit claustrum - study of the claustrocortical connections based on the retrograde axonal transport of fluorescent tracers. Brain Res 784:199–209. doi:10.1016/S0006-8993(97)01326-7
Kowianski P, Morys J, Wojcik S (2002) Postnatal development of NOS-ir neurons in the rat claustrum. Folia Morphol 61:11–17
Kowianski P, Morys JM, Wojcik S et al (2003) Co-localization of NOS with calcium-binding proteins during the postnatal development of the rat claustrum. Folia Morphol (Warsz) 62:211–214
Kowianski P, Morys JM, Wojcik S et al (2004) Neuropeptide-containing neurons in the endopiriform region of the rat: morphology and colocalization with calcium-binding proteins and nitric oxide synthase. Brain Res 996:97–110. doi:10.1016/j.brainres.2003.10.020
Krushkov I, Markova T, Tsankov A (1996) The participation of nitric oxide in the functions of the central nervous system and the cardiovascular system. Mol Med (Sofia) 1:21–27
Kullo IJ, Schwartz RS, Pompili VJ et al (1997) Expression and function of recombinant endothelial NO synthase in coronary artery smooth muscle cells. Arterioscler Thromb Vasc Biol 11:2405–2412
Kunzle H (1975) Bilateral projection from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res 88:195–209. doi:10.1016/0006-8993(75)90384-4
Kunzle H (1978) An autoradiographic analysis of the efferent connections from the premotor and adjacent prefrontal regions (area 6 and 9) in Macaca fascicularis. Brain Behav Evol 15:185–234
Landau E (1923) Zur kenntnis der Beziehungen des claustrums zum nucleus amygdalae und zur area piriformis im speziellen zum tractus olfactorius. Schweiz Arch Neurol Psychiatr 13:391–400
LeVay S, Sherk H (1981a) The visual claustrum of the cat. I. Structure and connections. J Neurosci 1:956–980
LeVay S, Sherk H (1981b) The visual claustrum of the cat. II. The visual field map. J Neurosci 1:981–992
Lieberman AR (1973) Neurons with presynaptic perikarya and presynaptic dendrites in the rat lateral geniculate nucleus. Brain Res 59:35–59. doi:10.1016/0006-8993(73)90252-7
Lin L-H, Talman T (2000) N-methyl-D-aspartate receptors on neurons that synthesize nitric oxide in rat nucleus tractus solitarii. Neuroscience 100:581–588. doi:10.1016/S0306-4522(00)00314-6
Loo TT (1931) The forebrain of the opossum, Didelphis virginiana. J Comp Neurol 52:1–148. doi:10.1002/cne.900520102
Lysakowski A, Singer M (2000) Nitric oxide synthase localized in a subpopulation of vestibular efferents in NADPH diaphorase histochemistry and nitric oxide synthase immunohistochemistry. J Comp Neurol 427:508–521. doi :10.1002/1096-9861(20001127)427:4<508::AID-CNE2>3.0.CO;2-L
Macchi G (1984) Morphology and structure of human claustrum. Cervello 24:1–26
Macchi G, Bentivoglio M, Minciacchi D et al (1983) Claustroneocortical projections studied in the cat by means of multiple retrograde fluorescent tracing. J Comp Neurol 215:121–134. doi:10.1002/cne.902150202
Maeda M, Inoue M, Takao S, Nakai M et al (1999) Central control mechanisms of circulation in the medulla oblongata by nitric oxide. Jpn J Physiol 49:467–478. doi:10.2170/jjphysiol.49.467
Mamos L (1984) Morphology of claustral neurons in the rat. Folia Morphol (Warsz) 43:73–78
Marino J, Cudeiro J (2003) Nitric oxide-mediated cortical activation: a diffuse wake-up system. J Neurosci 23:4299–4307
Martinelli G, Fridrich V, Holstein G (2002) l-citrulline immunostaining identifies nitric oxide production sites within neurons. Neuroscience 114:111–122. doi:10.1016/S0306-4522(02)00238-5
Mizukawa K (1990) Reduced nicotinamide-adenine-dinucleotide-phosphat-diaphorase histochemistry: light and electron microscopic investigations. Methods Neurosci 3:457–472
Mizukawa K, Vincent SR, McGeer PL et al (1989) Distribution of reduced-nicotinamide-adenine-dinucleotide-phosphat-diaphorase-positive cells and fibers in the cat neurvous system. J Comp Neurol 279:281–311. doi:10.1002/cne.902790210
Moreno-Lopez B, Escudero M, Delgado-Garcia JM et al (1996) Nitric oxide production by brain stem neurons is required for normal performance of eye movements in alert animals. Neuron 17:739–745. doi:10.1016/S0896-6273(00)80205-6
Moreno-Lopez B, Estrada C, Escudero M (1998) Mechanisms of action and targets of nitric oxide in the oculomotor system. J Neurosci 18:10672–10679
Moreno-Lopez B, Escudero M, Estrada C (2001) Morphological identification of nitric oxide sources and targets in the cat oculomotor system. J Comp Neurol 435:311–324. doi:10.1002/cne.1032
Morest DK (1971) Dendrodendritic synapses of the cells that have axons: the fine structure of the Golgi type II cells in the medial geniculate body of the cat. Z Anat Entwickl Gesch 133:216–246. doi:10.1007/BF00528025
Morys J, Berdel B, Maciejewska B et al (1996) Division of the human claustrum according to its architectonics, morphometric parameters and cortical connections. Folia Morphol (Warsz) 55:69–82
Neal JW, Pearson RCA, Powell TPS (1986) The relationship between the auditory cortex and the claustrum in the cat. Brain Res 366:145–151. doi:10.1016/0006-8993(86)91289-8
Norita M (1977) Demonstration of bilateral claustro-cortical connections in the cat with the method of retrograde axonal transport of horseradish peroxidase. Arch Histol Jpn 40:1–10
Olsen CR, Graybiel AM (1980) Sensory maps in the claustrum of the cat. Nature 288:479–481. doi:10.1038/288479a0
Otelin VA, Makarov FN (1972) Dokl Akad Nauk SSSR 202:723–725. Descending connections of the auditory cortex of the cat with contralateral neostriatal complex and claustrum, Ser Biol
Paloff AM (1985) Somatodendritic synapses in the central nucleus of colliculus inferior (CI) in the cat. J Hirnforsch 26:353–358
Paloff AM, Hinova-Palova DV (1998) Topographical distribution of NADPH-diaphorase-positive neurons in the cat’s inferior colliculus. J Hirnforsch 39:231–243
Paloff AM, Usunoff KG (1992a) The fine structure of the inferior colliculus in the cat. II. Synaptic organization. J Hirnforsch 32:77–106
Paloff AM, Usunoff KG (1992b) Projections to the inferior colliculus from the dorsal column nuclei. An experimental electron-microscopic study in the cat. J Hirnforsch 33:597–610
Paloff A, Bozhilova-Pastirova A, Hinova-Palova D, et al (1998) Co-existence of GABA and parvalbumin in the cat’s inferior colliculus. An electronmicroscopical study. Paper presented at the First congress of the Bulgarien Society for Neuroscience, Sofia, p 16
Paloff AM, Usunoff KG, Hinova-Palova D et al (1989) The fine structure of the inferior colliculus in the cat. I. Neuronal perikarya in the central nucleus. J Hirnforsch 30:69–90
Paloff A, Usunoff K, Yotovski P et al (2004) Parvalbumin-like immunostaining in the cat inferior colliculus. Light- and electron-microscopic investigation. Acta Histochem 106:219–234. doi:10.1016/j.acthis.2003.11.006
Papantchev V, Christova T, Paloff A et al (2003) NADPH diaphorase positive capillaries in the brain stem of the cat. Praemedicus Since 1925 22:126–131
Papantchev V, Paloff A, Christova T et al (2005) Light microscopical study of nitric oxide synthase I-positive neurons, including fibres in the vestibular nuclear complex of the cat. Acta Histochem 107:113–120. doi:10.1016/j.acthis.2005.01.004
Papantchev V, Paloff A, Hinova-Palova D et al (2006) Neuronal nitric oxide synthase immunopositive neurons in cat vestibular complex: a light and electron microscopic study. J Mol Histol 37:343–352. doi:10.1007/s10735-006-9061-6
Paxinos G, Watson C (1989) The rat brain in stereotaxic coordinates. Academic Press, New York
Pearson RCA, Brodal P, Gatter KC et al (1982) The organization of the connections between the cortex and the claustrum in the monkey. Brain Res 234:435–441. doi:10.1016/0006-8993(82)90883-6
Pilleri G (1961) The claustrum of Didelphis marsupialis Lin (Marsupialis, Didelphoidea). Acta Anat (Basel) 45:310–314. doi:10.1159/000141759
Pilleri G (1962) The claustrum of the Canadian beaver (Castor canadensis Kuhl): Structure and comparative anatomy. J Hirnforsch 5:59–81
Pro-Sistiaga P, Fuentes M, Alejo A et al (2002) Nitric oxide synthase expression in the rabbit claustrum (abstract) FENS forum, Paris
Rae ASL (1954) The connections of the claustrum. Confin Neurol Basel 14:211–219. doi:10.1159/000105714
Rahman F, Baizer J (2007) Neurochemically defined cell types in the claustrum of the cat. Brain Res 1159:94–111. doi:10.1016/j.brainres.2007.05.011
Real MA, Davila JC, Guirado S (2003) Expression of calcium-binding proteins in the mouse claustrum. J Chem Neuroanat 25:151–160. doi:10.1016/S0891-0618(02)00104-7
Reinozo-Suarez F (1961) Topographischer hirnatlas der katze fur experimental-physiologische untersuehungen. Darmstadt
Riche D, Lanoir J (1978) Some claustrocortical connections in the cat and baboon as studied by retrograde HRP transport. J Comp Neurol 177:435–444. doi:10.1002/cne.901770306
Rodrigo J, Springall DR, Uttenthal O et al (1994) Localization of nitric oxide synthase in the adult rat brain. Philos Trans R Soc Lond B Biol Sci 345:175–221. doi:10.1098/rstb.1994.0096
Romansky KV, Usunoff KG (1985) The fine structure of the subthalamic nucleus in the cat. I. Neuronal perikarya. J Hirnforsch 26:259–273
Rowniak M, Szteyn S, Robak A et al (1994) The types of neurons in the claustrum of Bison bonanus: Nissl and Golgi study. Folia Morphol 53:231–237
Sadowski M, Morys J, Jakubowska-Sadowska K (1997) Rat’s claustrum shows two main cortico-related zones. Brain Res 756:147–152
Samdani A, Dawson T, Dawson V (1997) Nitric oxide synthase in models of focal ischemia. Stroke 28:1283–1288
Sears CE, Ashley EA, Casadei B (2004) Nitric oxide control of cardiac function: is neuronal nitric oxide synthase a key component? Philos Trans R Soc Lond B Biol Sci 359:1021–1044. doi:10.1098/rstb.2004.1477
Seidel B, Stanarius A, Wolf G (1997) Differential expression of neuronal and endothelial nitric oxide synthase in blood vessels of the rat brain. Neurosci Lett 239:109–112. doi:10.1016/S0304-3940(97)00912-9
Seyidova D, Aliyev A, Rzayev N et al (2004) The role of nitric oxide in the pathogenesis of brain lesions during the development of Alzheimer’s disease. In vivo 18:325–333
Sherk H (1986) The claustrum and the cerebral cortex. In: Jones EG, Peters A (eds) Cerebral cortex, vol 5. Plenum Press, New York, pp 467–499
Sloniewski P, Usunoff KG, Pilgrim C (1986) Diencephalic and mesencephalic afferents of the rat claustrum. Anat Embryol (Berl) 173:401–411. doi:10.1007/BF00318925
Soares JG, Mendez-Otero R, Gattass R (2003) Distribution of NADPH-diaphorase in the superior colliculus of Cebus monkeys, and co-localization with calcium-binding proteins. Neurosci Res 46:475–483. doi:10.1016/S0168-0102(03)00125-1
Spahn B, Braak H (1985) Percentage of projection neurons and various types of interneurons in the human claustrum. Acta Anat (Basel) 122:245–248
Stelmasiak M (1955) Volume of the claustrum in man. Folia Morphol (Warsz) 6:137–144
Sugiyama T, Fujita M, Koide N et al (2003) Differences in the mechanism of nitric oxide production between mouse vascular endothelial cells and macrophages. J Endotoxin Res 9:108–112
Tanne-Gariepy J, Boussaoud D, Rouiller EM (2002) Projections of the claustrum to the primary motor, premotor, and prefrontal cortices in the macaque monkey. J Comp Neurol 454:140–157. doi:10.1002/cne.10425
Usunoff KG (1990) Cytoarchitectural, ultrastructural and histochemical characteristics of substantia nigra, Thesis. Medical Academy, Sofia, Bulgaria
Vater M, Braun K (1994) Parvalbumin, calbindin D-28 k, and calretinin immunoreactivity in the ascending auditory pathway of horseshoe bats. J Comp Neurol 341:534–558. doi:10.1002/cne.903410409
Wada K, Chatzipanteli K, Kraydieh S et al (1998) The role of inducible nitric oxide synthase in the pathophysiology of traumatic brain injury in the rat. Neurosurgery 43:1427–1436. doi:10.1097/00006123-199812000-00096
Witter MP, Room P, Groenewegen HJ et al (1988) Reciprocal connections of the insular and piriform claustrum with limbic cortex: an anatomical study in the cat. Neurosci 24:519–539. doi:10.1016/0306-4522(88)90347-8
Wojcik S, Dziewiatkowski J, Spodnik E et al (2004) Analysis of calcium-binding protein immunoreactivity in the claustrum and the endopiriform nucleus of the rabbit. Acta Neurobiol Exp (Warsz) 64:449–460
Zilles K, Zilles B (1980) Schleicher A. A quantitative approach to cytoarchitectonics. VI. The areal pattern of the cortex of the albino rat. Anat Embryol (Berl) 159:335–360. doi:10.1007/BF00317655
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hinova-Palova, D., Edelstein, L., Paloff, A. et al. Neuronal nitric oxide synthase immunopositive neurons in cat claustrum—a light and electron microscopic study. J Mol Hist 39, 447–457 (2008). https://doi.org/10.1007/s10735-008-9184-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-008-9184-z