Abstract
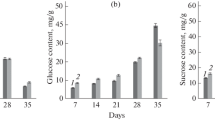
We previously reported that expression and activity of acid invertases (AI) are increased in peach fruit under chilling stress. In order to determine which AI genes respond to chilling stress, seven AI genes, two vacuolar invertases (VINs) and five cell wall-bound invertases (CWINs), were identified and cloned. The predicted amino acid sequences of the genes contain conserved sites characteristic of plant AIs such as NDPNG/A, the sucrose-binding site, and MWECV/P, a cysteine catalytic motif. Using gene-specific primers, the expression of each gene was measured in ‘Baifeng’ and ‘Yulu’ peach fruits stored at 0, 5, 10 and 20 °C. Of the seven genes, expression of PpVIN2 was the most affected by chilling stress; the largest increases were in fruit stored at 5 °C, up to 17-fold in ‘Baifeng’ fruit, and up to 280-fold in ‘Yulu’ fruit. Overall, VIN activity was much higher than CWIN activity in stored peach fruit. In both cultivars reducing sugar content increased significantly and sucrose content decreased gradually during storage at 5 °C relative to other temperatures, and was accompanied by severe chilling injury symptoms. Thus, PpVIN2 appears to be induced by chilling and may play an important role in sucrose metabolism in peach fruit subjected to cold storage.






Similar content being viewed by others
References
Abidi W, Cantín CM, Jiménez S, Giménez R, Moreno M, Gogorcena Y (2015) Influence of antioxidant compounds, total sugars and genetic background on the chilling injury susceptibility of a non-melting peach (Prunus persica (L.) Batsch) progeny. J Sci Food Agric 95:351–358
Bhaskar PB, Wu L, Busse JS, Whitty BR, Hamernik AJ, Jansky SH, Buell CR, Bethke PC, Jiang J (2010) Suppression of vacuolar invertase gene prevents cold-induced sweetening in potato. Plant Physiol 154:939–948
Bocock PN, Morse AM, Dervinis C, Davis JM (2007) Evolution and diversity of invertase genes in Populus trichocarpa. Planta 227:565–576
Bournay AS, Hedley PE, Maddison A, Waugh R, Machray GC (1996) Exon skipping induced by cold stress in a potato invertase gene transcript. Nucleic Acids Res 24:2347–2351
Colaric M, Veberic R, Stampar F, Hudina M (2005) Evaluation of peach and nectarine fruit quality and correlations between sensory and chemical attributes. J Sci Food Agric 85:2611–2616
Ding Z, Tian S, Meng X, Xu Y (2009) Hydrogen peroxide is correlated with browning in peach fruit stored at low temperature. Front Chem Eng China 3(4):363–374
Draffehn AM, Meller S, Li L, Gebhardt C (2010) Natural diversity of potato (Solanum tuberosum) invertases. BMC Plant Biol 10:271
Godt DE, Roitsch T (1997) Regulation and tissue-specific distribution of mRNAs for three extracellular invertase isoenzymes of tomato suggests an important function in establishing and maintaining sink metabolism. Plant Physiol 115:273–282
Goetz M, Roitsch T (1999) The different pH optima and substrate specificities of extracellular and vacuolar invertases from plants are determined by a single amino-acid substitution. Plant J 20:707–711
Hirose T, Takano M, Terao T (2002) Cell wall invertase in developing rice caryopsis: molecular cloning of OsCIN1 and analysis of its expression in relation to its role in grain filling. Plant Cell Physiol 43:452–459
Hothorn M, Wolf S, Aloy P, Greiner S, Scheffzek K (2004) Structural insights into the target specificity of plant invertase and pectin methyl-esterase inhibitory proteins. Plant Cell 16:3437–3447
Hsien C, Liu L, Yeh S, Chen C, Sung H (2006) Molecular cloning and functional identification of invertase isoenzymes from green bamboo Bambusa oldhamii. J Agric Food Chem 54:3101–3107
Hu PZ, Ju YW, Gai HQ, Gai FY, Kai JQ, Li FW, Shao LZ (2014) The role of sucrose-metabolizing enzymes in pear fruit that differ in sucrose accumulation. Acta Physiol Plant 36:71–77
Itai A, Tanahashi T (2008) Inhibition of sucrose loss during cold storage in Japanese pear (Pyrus pyrifolia Nakai) by 1-MCP. Postharvest Biol Technol 48:355–363
Itai A, Hatanaka R, Irie H (2015) Effects of storage temperature on fruit quality and expression of sucrose phosphate synthase and acid invertase genes in Japanese pear. Horticul J 84:227–232
Ji X, Ende WVD, Laere AV, Cheng S, Bennett J (2005) Structure, evolution, and expression of the two invertase gene families of rice. J Mol Evol 60:615–634
Jin Y, Ni DA, Ruan YL (2011) Posttranslational elevation of cell wall invertase activity by silencing its inhibitor in tomato delays leaf senescence and increases seed weight and fruit hexose level. Plant Cell 21:2072–2089
Link M, Rausch T, Greiner S (2004) In Arabidopsis thaliana, the invertase inhibitors AtC/VIF1 and 2 exhibit distinct target enzyme specificities and expression profiles. FEBS Lett 573:105–109
Liu X, Zhang C, Ou Y, Lin Y, Song B, Xie C, Liu J, Li XQ (2011) Systematic analysis of potato acid invertase genes reveals that a cold-responsive member, StvacINV1, regulates cold-induced sweetening of tubers. Mol Genet Genom 286:109–118
Liu X, Lin Y, Liu J, Song B, Ou Y, Zhang H, Li M, Xie C (2013) StInvInh2 as an inhibitor of StvacINV1 regulates the cold-induced sweetening of potato tubers by specifically capping vacuolar invertase activity. Plant Biotechnol J 11:640–647
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Matsuuraendo C, Kobayashi A, Noda T, Takigawa S, Yamauchi H, Mori M (2004) Changes in sugar content and activity of vacuolar acid invertase during low-temperature storage of potato tubers from six Japanese cultivars. J Plant Res 117:131–137
Mckenzie MJ, Sowokinos JR, Shea IM, Gupta SK, Lindlauf RR, Anderson JAD (2005) Investigations on the role of acid invertase and UDP-glucose pyrophosphorylase in potato clones with varying resistance to cold-induced sweetening. Am J Potato Res 82:231–239
Mckenzie MJ, Chen RK, Harris JC, Ashworth MJ, Brummell DA (2013) Post-translational regulation of acid invertase activity by vacuolar invertase inhibitor affects resistance to cold-induced sweetening of potato tubers. Plant Cell Environ 36:176–185
Miron D, Schaffer AA (1990) Sucrose phosphate synthase, sucrose synthase, and invertase activities in development fruit of Lycopersiconesculentum Mill. and the sucrose accumulating Lycopersicon hirsutum Humb. and Bonpl. Plant Physiol 95:623–627
Moriguchi T, Sanada T, Yamaki S (1990) Seasonal fluctuations of some enzymes relating to sucrose and sorbitol metabolism in peach fruit. J Am Soc Hortic Sci 115:278–281
Palmer W, Ru L, Jin Y, Patrick J, Ruan YL (2015) Tomato ovary-to-fruit transition is characterized by a spatial shift of mRNAs for cell wall invertase and its inhibitor with the encoded proteins localized to sieve elements. Mol Plant 8:315–328
Proels RK, Hückelhoven R (2014) Cell-wall invertases, key enzymes in the modulation of plant metabolism during defence responses. Mol Plant Pathol 15:858–864
Qin G, Zhu Z, Wang W, Cai J, Chen Y, Li L, Tian S (2016) A tomato vacuolar invertase inhibitor mediates sucrose metabolism and influences fruit ripening. Plant Physiol 172:1596–1611
Rausch T, Greiner S (2004) Plant protein inhibitors of invertases. Biochim Biophys Acta 1696:253–261
Richardson DL, Davies HV, Ross HA, Mackay GR (1990) Invertase activity and its relation to hexose accumulation in potato tubers. J Exp Bot 41:95–99
Roitsch T, González MC (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9:606–613
Ruan YL, Jin Y, Yang YJ, Li GJ, Boyer JS (2010) Sugar input, metabolism, and signaling mediated by invertase: roles in development, yield potential, and response to drought and heat. Mol Plant 03:942–955
Schaffer AA, Rylski I, Fogelman M (1989) Carbohydrate content and sucrose metabolism in developing Solanum muricatum fruits. Phytochem 28:737–739
Shao X, Zhu Y, Cao S, Wang H, Song Y (2013) Soluble sugar content and metabolism as related to the heat-induced chilling tolerance of loquat fruit during cold storage. Food Bioprocess Technol 6:3490–3498
Sherson SM, Alford HL, Forbes SM, Wallace G, Smith SM (2003) Roles of cell-wall invertases and monosaccharide transporters in the growth and development of Arabidopsis. J Exp Bot 54:525–531
Sturm A (1996) Molecular characterization and functional analysis of sucrose-cleaving enzymes in carrot (Daucus carota L.). J Exp Bot 47:1187–1192
Sturm A (1999) Invertases. primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol 121:1–7
Tauzin AS, Sulzenbacher G, Lafond M, Desseaux V, Reca IB, Perrier J, Bellincampi D, Fourquet P, Lévêque C, Giardina T (2014) Functional characterization of a vacuolar invertase from Solanum lycopersicum: post-translational regulation by N-glycosylation and aproteinaceous inhibitor. Biochimie 101:39–49
Valero D, Serrano M, Riquelme F (1997) Polyamines, ethylene, and physicochemical changes in low-temperature-stored peach (Prunus persica L. Cv. Maycrest). J Agric Food Chem 45:3406–3410
Veillet F, Gaillard C, Coutos-Thevenot P, La Camera S (2016) Targeting the AtCWIN1 Gene to explore the role of invertases in sucrose transport in roots and during Botrytis cinerea infection. Front Plant Sci 7:1899
Verde I, Abbott AG, Scalabrin S (2013) The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat Genet 45:487–494
Wan H, Wu L, Yang Y, Zhou G, Ruan U (2017) Evolution of sucrose metabolism: the dichotomy of invertases and beyond. Trends in Plant Sci 11:1–15
Wang K, Shao XF, Gong YF, Zhu Y, Wang HF, Zhang XL, Yu DD, Yu F, Qiu ZY, Lu H (2013) The metabolism of soluble carbohydrates related to chilling injury in peach fruit exposed to cold stress. Postharvest Biol Technol 86:53–61
Xu CA, Coleman WK, Meng FR, Bonierbale M, Li XQ (2009) Relationship between glucose accumulation and activities of acid invertase and its inhibitors in potatoes under simulated commercial conditions. Potato J 36:35–44
Yu F, Ni ZM, Shao XF, Yu LN, Liu HX, Xu F, Wang HF (2015) Differences in sucrose metabolism in peach fruit stored at chilling stress versus nonchilling stress temperatures. HortScience 50:1542–1548
Yu LN, Liu HX, Shao XF, Yu F, Wei YY, Ni ZM, Xu F, Wang HF (2016) Effects of hot air and methyl jasmonate treatment on the metabolism of soluble sugars in peach fruit during cold storage. Postharvest Biol Technol 113:8–16
Yu LN, Shao XF, Wei YY, Xu F, Wang HF (2017) Sucrose degradation is regulated by 1-methycyclopropene treatment and is related to chilling tolerance in two peach cultivars. Postharvest Biol Technol 124:25–34
Zhang CF, Ding ZS, Xu XB, Wang Q, Qin GZ, Tian SP (2010) Crucial roles of membrane stability and its related proteins in the tolerance of peach fruit to chilling injury. Amino Acid 39(1):181–194
Zhang C, Shen Z, Zhang Y, Han J, Ma R, Korir NK, Yu M (2012) Cloning and expression of genes related to the sucrose-metabolizing enzymes and carbohydrate changes in peach. Acta Physiol Plant 35:589–602
Zhong C, Kai G, Su X, Rao P, An X (2015) Genome-wide identification of the invertase gene family in Populus. PLoS ONE 10:304–315
Zhu X, Richael C, Chamberlain P, Busse JS, Bussan AJ, Jiang J, Bethke PC (2014) Vacuolar invertase gene silencing in potato (Solanum tuberosum L.) improves processing quality by decreasing the frequency of sugar-end defects. PLoS ONE 9:1–11
Acknowledgements
This study was sponsored by the National Natural Science Foundation of China (No. 31671903), the Nature Science Foundation of Zhejiang Province (No. LR15C200002), and the K. C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, X., Wei, Y., Kou, J. et al. PpVIN2, an acid invertase gene family member, is sensitive to chilling temperature and affects sucrose metabolism in postharvest peach fruit. Plant Growth Regul 86, 169–180 (2018). https://doi.org/10.1007/s10725-018-0419-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-018-0419-z