Abstract
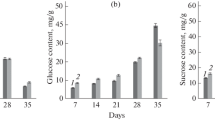
Since the carbohydrate content affects pear flavor during the process of growth, it is necessary to determine the sugar components that accumulate in the fruit. We analyzed the fruit carbohydrate content, and the gene expression and activity of acid invertase (AI), neutral invertase (NI), sucrose synthase (SS), and sucrose phosphate synthase (SPS) during the development of “Huangguan” and “Yali” pears. The results demonstrate that during development, the fruit sugar metabolism of the “Huangguan” pear follows a typical sorbitol–starch-soluble sugars middle model, whereas the “Yali” pear fruit follows a typical sorbitol–sucrose–starch-soluble sugars middle model. In the “Huangguan” pear, we found the AI and NI gene expressions, as well as AI (P < 0.05) and NI (P < 0.01) enzyme activities, to be positively correlated, whereas we found the NI gene expression and NI enzyme activity of “Yali” pear to be negatively correlated (P < 0.01). We observed the high levels of late-stage AI and early-stage SS during development to roughly correspond with the gene expression found in the late and early stages, respectively, suggesting their potential regulatory roles in “Huangguan” pear fruit development. Our results indicate that the primary function of SPS during the early developmental stage is to accumulate sucrose, whereas the primary function of AI is to promote hexose accumulation during the late developmental stage of mature “Yali” pear fruit.




Similar content being viewed by others
References
Yu LN, Liu HX, Shao XF et al (2016) Effects of hot air and methyl jasmonate treatment on the metabolism of soluble sugars in peach fruit during cold storage. Postharvest Biol Technol 113:8–16
Hu LS, Wu G, Hao CY et al (2016) Transcriptome and selected metabolite analyses reveal points of sugar metabolism in jackfruit (Artocarpus heterophyllus Lam.). Plant Sci 248:45–56
Yang ZY, Wang TD, Wang HC et al (2013) Patterns of enzyme activities and gene expressions in sucrose metabolism in relation to sugar accumulation and composition in the aril of Litchi chinensis Sonn. J Plant Physiol 170(8):731–740
Jiang N, Jin LF, da Silva JAT et al (2014) Activities of enzymes directly related with sucrose and citric acid metabolism in citrus fruit in response to soil plastic film mulch. Sci Hortic 168(3):73–80
Basson CE, Groenewald JH, Kossmann J et al (2010) Sugar and acid-related quality attributes and enzyme activities in strawberry fruits: invertase is the main sucrose hydrolysing enzyme. Food Chem 121(4):1156–1162
Choudhury SR, Roy S, Sengupta DN (2009) A comparative study of cultivar differences in sucrose phosphate synthase gene expression and sucrose formation during banana fruit ripening. Postharvest Biol Technol 54(1):15–24
Roitsch T, Gonzalez MC (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9(12):606–613
Manning K, Maw GA (1975) Distribution of acid invertase in the tomato plant. Phytochemistry 14(9):1965–1969
Moscatello S, Famiani F, Proietti S et al (2011) Sucrose synthase dominates carbohydrate metabolism and relative growth rate in growing kiwifruit (Actinidia deliciosa, cv Hayward). Sci Hortic 128(3):197–205
Bosch S, Grof CPL, Botha F (2004) Expression of neutral invertase in sugarcane. Plant Sci 166(5):1125–1133
Ruan YL, Jin Y, Yang YJ et al (2010) Sugar input, metabolism, and signaling mediated by invertase: roles in development, yield potential, and response to drought and heat. Mol Plant 3(6):942–955
Tanase K, Yamaki S (2000) Sucrose synthase isozymes related to sucrose accumulation during fruit development of Japanese pear (Pyrus pyrifolia Nakai). J Jpn Soc Hortic Sci 69(6):671–676
Lopez-Gomez R, Gomez-Lim MA (1992) A method for extracting intact RNA from fruits rich in polysaccharides using ripe mango mesocarp. HortScience 27(5):440–442
Nielsen TH, Skjærbæ HC, Karlsen P (1991) Carbohydrate metabolism during fruit development in sweet pepper (Capsicum annuum) plants. Physiol Plantarum 82(2):311–319
Mbéguié-A-Mbéguié D, Fils-Lycaon B, Chillet M (2008) Extraction and purification of total RNA from banana tissues (small scale). Fruits 63(4):255–262
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Fils-Lycaon B, Julianus P, Chillet M (2011) Acid invertase as a serious candidate to control the balance sucrose versus (glucose plus fructose) of banana fruit during ripening. Sci Hortic 129(2):197–206
Moriguchi T, Abe K, Sanada T et al (1992) Levels and role of sucrose synthase, sucrose-phosphate synthase, and acid invertase in sucrose accumulation in fruit of Asian pear. J Am Soc Hortic Sci 117(2):274–278
Chen JL, Wang ZF, Wu JH et al (2007) Chemical compositional characterization of eight pear cultivars grown in China. Food Chem 104(1):268–275
Zheng GQ, Zheng ZY, Xu X et al (2010) Variation in fruit sugar composition of Lycium barbarum L. and Lycium chinense Mill. of different regions and varieties. Biochem Syst Ecol 38(3):275–284
Nitsch J (1953) The physiology of fruit growth. Annu Rev Plant Physiol 4(4):199–236
Mwaniki MW, Mathooko FM, Matsuzaki M et al (2005) Expression characteristics of seven members of the beta-galactosidase gene family in ‘La France’ pear (Pyrus communis L.) fruit during growth and their regulation by 1-methylcyclopropene during postharvest ripening. Postharvest Biol Technol 36(3):25–263
Beruter J (2004) Carbohydrate metabolism in two apple genotypes that differ in malate accumulation. J Plant Physiol 161(9):1011–1029
Klann EM, Chetelat RT, Bennett AB (1993) Expression of acid invertase gene controls sugar composition in tomato (Lycopersicon) fruit. Plant Physiol 103(3):863–870
Miron D, Schaffer AA (1991) Sucrose phosphate synthase, sucrose synthase and acid invertase activities in developing tomato fruit of Lycopersicon esculentum Mill. and the sucrose accumulating Lycopersicon hirsutum Humb. and Bonpl. Plant Physiol 95(2):623–627
Giorno F, Guerriero G, Biagetti M et al (2013) Gene expression and biochemical changes of carbohydrate metabolism in in vitro micro-propagated apple plantlets infected by ‘Candidatus Phytoplasma mali’. Plant Physiol Biochem 70:311–317
Kajiura I, Yamaki S, Omura M et al (1979) Improvement of sugar content and composition in fruits, and classifications of East Asian pears by the principal component analysis of sugar compositions in fruits. Jpn J Breed 29(1):1–12
Wang L, Cui N, Zhao XC et al (2014) Accumulation of carbohydrate and regulation of 14-3-3 protein on sucrose phosphate synthase (SPS) activity in two tomato species. J Integr Agric 13(2):358–364
Sturm A, Tang GQ (1999) The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends Plant Sci 4(10):401–407
Yelle S, Chetelat RT, Dorais M et al (1991) Sink metabolism in tomato fruit: IV. Genetic and biochemical analysis of sucrose assimilation. Plant Physiol 95(4):1026–1035
Sun JD, Loboda T, Sung SJS et al (1992) Sucrose synthase in wild tomato, Lycopersicon chmielewskii, and tomato fruit sink strength. Plant Physiol 98(3):1163–1169
Acknowledgements
This work was funded as a key project in the National Science and Technology Pillar Program during the 11th 5-Year Plans (No. 2006BAD22B01). This work was also supported by the National Natural Science Foundation of China (No. 31171769) and the Special Fund for Agro-Scientific Research in the Public Interest (No. 201303075). We are very grateful to Dr. Shijie Yan (Tianjin Agricultural University) for providing the cold room.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kou, X., Li, Y., Zhang, Y. et al. Gene Expression and Activity of Enzymes Involved in Sugar Metabolism and Accumulation During “Huangguan” and “Yali” Pear Fruit Development. Trans. Tianjin Univ. 24, 101–110 (2018). https://doi.org/10.1007/s12209-017-0104-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-017-0104-8