Abstract
LEA (late embryogenesis abundant) proteins are firstly discovered in seeds and then identified in vegetative tissues of different plant species. They are mainly regulated under abiotic stress conditions. Although genome wide studies of different gene family members have been performed in cucumber, there is no such a study for LEA genes. We have identified 79 LEA genes in the cucumber genome. Based on phylogenetic analysis, CsLEA genes could be classified into seven groups in which structural motifs are relatively conserved. Tandem duplications play an important role in cucumber genome for LEA gene expansion. Orthologous and chromosomal relationships of CsLEA genes were observed based on comparative mapping analysis with other species. The in silico micro-RNA (miRNA) target analyses indicated that 37 CsLEA genes were targeted by different miRNAs, especially mir854 and mir414 are the most abundant identified ones. Public available RNA-seq data were analyzed for expression analysis of CsLEA genes in different tissues of cucumber. According to genome-wide expression analysis, nine CsLEA genes showed higher expression profiles in all tissues. The expression profiles of ten CsLEA genes in the root and leaf tissues of drought-stressed cucumber were examined using qRT-PCR. Among them, CsLEA-54 induced after stress application in leaf and root tissues and might provide adaptation to drought stress for cucumber. CsLEA-09, CsLEA-32 and CsLEA-57 genes responded to drought after 3 h later and might be considered as early response genes to water limitation. This research could help us to improve understanding of contribution of CsLEAs to drought tolerance in cucumber.






Similar content being viewed by others
References
Ambros V, Chen X (2007) The regulation of genes and genomes by small RNAs. Development 134(9):1635–1641
Arteaga-Va´zquez M, Caballero-Pe´rez J, Vielle-Calzada JP (2006) A family of microRNAs present in plants and animals. Plant Cell 18:3355–3369
Bailey TL, Elkan C (1994) Fitting a mixture model by expectation maximization to discover motifs in biopolymers. In: Proceedings of the second international conference on intelligent systems for molecular biology, AAAI Press, Menlo Park, California, pp 28–36
Baloglu MC (2014) Genome-wide in silico identification and comparison of Growth Regulating Factor (GRF) genes in Cucurbitaceae family. Plant Omics J 7:260–270
Baloglu MC, Eldem V, Hajyzadeh M, Unver T (2014) Genome-wide analysis of the bZIP transcription factors in cucumber. PLoS One 9(4):e96014. doi:10.1371/journal.pone.0096014
Baloglu MC, Ulu F, Altunoglu YC, Pekol S, Alagoz G, Ese O (2015) Identification, molecular characterization and expression analysis of RPL24 genes in three Cucurbitaceae family members: cucumber, melon and watermelon. Biotechnol Biotechnol Equip 26(6):1024–1034
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Battaliga M, Olvera-Carrillo Y, Garciarrubio A, Campos F, Covarrubias AA (2008) The enigmatic LEA proteins and other hydrophilins. Plant Physiol 148:6–24
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acids Res 28:235–242
Boswell LC, Menze MA, Hand SC (2009) Multiple isoforms of late embryogenesis abundant proteins in Artemia franciscana embryos. Integr Comp Biol 49:E203
Bray EA (1993) Molecular responses to water deficit. Plant Physiol 103:1035–1040
Cai X, Ye J, Hu T, Zhang Y, Ye Z, Li H (2014) Genome-wide classification and expression analysis of nucleobase–ascorbate transporter (NAT) gene family in tomato. Plant Growth Regul 73(1):19–30
Cao J, Li X (2015) Identification and phylogenetic analysis of late embryogenesis abundant proteins family in tomato (Solanum lycopersicum). Planta 241(3):757–772
Cao J, Shi F, Liu X, Huang G, Zhou M (2012) Phylogenetic analysis and evolution of aromatic amino acid hydroxylase. FEBS Lett 584:4775–4782
Caraux G, Pinloche S (2005) PermutMatrix: a graphical environment to arrange gene expression profiles in optimal linear order. Bioinform Appl Note 21(7):1280–1281
Charfeddine S, Saïdi MN, Charfeddine M, Gargouri-Bouzid R (2015) Genome-wide identification and expression profiling of the late embryogenesis abundant genes in potato with emphasis on dehydrins. Mol Biol Rep 42(7):1163–1174
Chen Y, Cao J (2014) Comparative genomic analysis of the Sm gene family in rice and maize. Gene 539:238–249
Conesa A, Götz S (2008) Blast2GO: a comprehensive suite for functional analysis in plant genomics. Int J Plant Genom. doi:10.1155/2008/619832
Dalal M, Tayal D, Chinnusamy V, Bansal KC (2009) Abiotic stress and ABA-inducible group 4 LEA from Brassica napus plays a key role in salt and drought tolerance. J Biotechnol 139(2):137–145. doi:10.1016/j.jbiotec.2008.09.014
Denekamp NY, Reinhardt R, Kube M, Lubzens E (2010) Late Embryogenesis Abundant (LEA) proteins in nondesiccated, encysted, and diapausing embryos of rotifers. Biol Reprod 82(4):714–724. doi:10.1095/biolreprod.109.081091
Du D, Zhang Q, Cheng T et al (2013) Genome-wide identification and analysis of late embryogenesis abundant (LEA) genes in Prunus mume. Mol Biol Rep 40(2):1937–1946. doi:10.1007/s11033-012-2250-3
Dure LI, Galau GA (1981) Developmental biochemistry of cottonseed embryogenesis and germination XIII. Regulation of biosynthesis of principal storage proteins. Plant Physiol 68(1):187–194
Dure L, Crouch M, Harada J, Ho THD, Mundy J, Quatrano R, Thomas T, Sung ZR (1989) Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol Biol 12(5):475–486
Eichinger L, Pachebat JA, Glockner G, Rajandream MA, Sucgang R, Berriman M (2005) The genome of the social amoeba Dictyostelium discoideum. Nature 435:43–57
Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5(5):199–206
Filiz E, Ozyigit II, Tombuloglu H, Koc I (2013) In silico comparative analysis of LEA (Late Embryogenesis Abundant) proteins in Brachypodium distachyon L. Plant Omics 6(6):433
Gal TZ, Glazer I, Koltai H (2004) An LEA group 3 family member is involved in survival of C. elegans during exposure to stress. FEBS Lett 577:21–26
George S, Usha B, Parida A (2009) Isolation and characterization of an atypical LEA protein coding cDNA and its promoter from drought-tolerant plant Prosopis juliflora. Appl Biochem Biotechnol 157(2):244–253. doi:10.1007/s12010-008-8398-6
Goodstein DM, Shu S, Howson R et al (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 40:D1178–D1186. doi:10.1093/nar/gkr944
Goyal K, Walton LJ, Tunnacliffe A (2005) LEA proteins prevent protein aggregation due to water stress. Biochem J 388:151–157
Grelet J, Benamar A, Teyssier E et al (2005) Identification in pea seed mitochondria of a late-embryogenesis abundant protein able to protect enzymes from drying. Plant Physiol 137:157–167
Guleria P, Yadav SK (2011) Identification of miR414 and expression analysis of conserved miRNAs from Stevia rebaudiana. Genom Proteom Bioinform 9(6):211–217
Guo AY, Zhu QH, Chen X, Luo JC (2007a) GSDS: a gene structure display server. Yi chuan Hereditas/Zhongguo yi chuan xue hui bian ji 29(8):1023–1026
Guo Q, Xiang A, Yang Q, Yang Z (2007b) Bioinformatic identification of microRNAs and their target genes from Solanum tuberosum expressed sequence tags. Chin Sci Bull 52(17):2380–2389
Gurley WB (2000) HSP101: a key component for the acquisition of thermotolerance in plants. Plant Cell 12(4):457–460
Hanin M, Brini F, Ebel C, Toda Y, Takeda S, Masmoudi K (2011) Plant dehydrins and stress tolerance. Plant Signal Behav 6:1503–1509
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Stn Circ 347:1–32
Huang S, Li R, Zhang Z et al (2009) The genome of the cucumber Cucumis sativus L. Nat Genet 41(12):1275–1281
Hunault G, Jaspard E (2010) LEAPdb: a database for the late embryogenesis abundant proteins. BMC Genom 11:221. doi:10.1186/1471-2164-11-221
Hundertmark M, Hincha DK (2008) LEA (Late Embryogenesis Abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom. doi:10.1186/1471-2164-9-118
Jakoby M, Weisshaar B, Dröge-Laser W, Vicente-Carbajosa J, Tiedemann J, Kroj T, Parcy F (2002) bZIP transcription factors in Arabidopsis. Trends Plant Sci 7(3):106–111
Jimenez AJ, Alonso-Ramirez A, Nicolas C (2008) Two cDNA clones (FsDhn1 and FsClo1) up-regulated by ABA are involved in drought responses in Fasus sylvatica L seeds. J Plant Physiol 165(17):1798–1807. doi:10.1016/j.jplph.2007.11.013
Jones-Rhoades MW, Bartel DP, Bartel B (2006) MicroRNAs and their regulatory roles in plants. Annu Rev Plant Biol 57:19–53. doi:10.1146/annurev.arplant.57.032905.105218
Kelley LA, Sternberg MJE (2009) Protein structure prediction on the web: a case study using the Phyre server. Nat Protoc 4:363–371
Kikawada T, Nakahara H, Kanamori Y, Iwata K, Watanabe M, McGee B, Tunnacli A, Okuda T (2006) Dehydration induced expression of LEA proteins in an anhydrobiotic chironomid. Biochem Biophys Res Commun 348:56–61
Kondrashov FA, Rogozin IB, Wolf YI, Koonin EV (2002) Selection in the evolution of gene duplications. Genome Biol 3(2):research0008.1–research0008.9
Kosová K, Vítámvás P, Prášil IT (2007) The role of dehydrins in plant response to cold. Biol Plant 51(4):601–617
Lambermon MH, Simpson GG, Wieczorek Kirk DA, Hemmings- Mieszczak M, Klahre U, Filipowicz W (2000) UBP1, a novel hnRNP-like protein that functions at multiple steps of higher plant nuclear pre-mRNA maturation. EMBO J 19:1638–1649
Lan T, Gao J, Zeng QY (2013) Genome-wide analysis of the LEA (late embryogenesis abundant) protein gene family in Populus trichocarpa. Tree Genet Genomes 9(1):253–264
Letunic I, Doerks T, Bork P (2012) SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res 40(D1):D302–D305. doi:10.1093/nar/gkr931
Li X, Cao J (2016) Late Embryogenesis Abundant (LEA) Gene Family in Maize: identification, evolution, and expression profiles. Plant Mol Biol Report 34(1):15–28
Li L, Xu HL, Yang XL, Li YX, Hu YK (2011) Genome-wide identification, classification and expression analysis of LEA gene family in soybean. Sci Agric Sin 44(19):3945–3954
Li Q, Zhang C, Li J, Wang L, Ren Z (2012) Genome-wide identification and characterization of R2R3MYB family in Cucumis sativus. Plos One 7(10):e47576
Ling J, Jiang W, Zhang Y et al (2011) Genome-wide analysis of WRKY gene family in Cucumis sativus. BMC Genom 12(1):471
Liu Y, Zheng Y, Zhang Y, Wang W, Li R (2010) Soybean PM2 protein (LEA3) confers the tolerance of Escherichia coli and stabilization of enzyme activity under diverse stresses. Curr Microbiol 60:373–378
Liu Y, Jiang H, Chen W, Qian Y, Ma Q, Cheng B, Zhu S (2011) Genome-wide analysis of the auxin response factor (ARF) gene family in maize (Zea mays). Plant Growth Regul 63(3):225–234
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Lough TJ, Lucas WJ (2006) Integrative plant biology: role of phloem long-distance macromolecular trafficking. Annu Rev Plant Biol 57:203–232
Lv J, Rao J, Johnson F, Shin S, Zhu Y (2015) Genome-wide identification of jasmonate biosynthetic genes and characterization of their expression profiles during apple (Malus × domestica) fruit maturation. Plant Growth Regul 75(1):355–364
Lynch M, Conery JS (2000) The evolutionary fate and consequences of duplicate genes. Science 290:1151–1155. doi:10.1126/science.290.5494.1151
Marunde MR, Samarajeewa DA, Anderson J, Li S, Hand SC, Menze MA (2013) Improved tolerance to salt and water stress in Drosophila melanogaster cells conferred by late embryogenesis abundant protein. J Insect Physiol 59(4):377–386
Mehan MR, Freimer NB, Ophoff RA (2004) A genome-wide survey of segmental duplications that mediate common human genetic variation of chromosomal architecture. Hum Genom 1(5):335–344
Olvera-Carrillo Y, Campos F, Luis Reyes J, Garciarrubio A, Covarrubias AA (2010) Functional analysis of the group 4 late embryogenesis abundant proteins reveals their relevance in the adaptive response during water deficit in Arabidopsis. Plant Physiol 154(1):373–390. doi:10.1104/pp.110.158964
Oztur ZN, Talame V, Deyholos M, Michalowski CB, Galbraith DW, Gozukirmizi N, Tuberosa R, Bohnert HJ (2002) Monitoring large-scale changes in transcript abundance in drought- and salt stressed barley. Plant Mol Biol 48(5–6):551–573. doi:10.1023/a:1014875215580
Park SC, Kim YH, Jeong JC, Kim CY, Lee HS, Bang JW, Kwak SS (2011) Sweetpotato late embryogenesis abundant 14 (IbLEA14) gene influences lignification and increases osmotic and salt stress-tolerance of transgenic calli. Planta 233(3):621–634. doi:10.1007/s00425-010-1326-3
Quevillon E, Silventoinen V, Pillai S et al (2005) InterProScan: protein domains identifier. Nucleic Acids Res 33:W116–W120. doi:10.1093/nar/gki442
Reyes JL, Campos F, Wei H, Arora R, Yang Y, Karlson DT, Covarrubias AA (2008) Functional dissection of hydrophilins during in vitro freeze protection. Plant Cell Environ 31:1781–1790
Romanel EA, Schrago CG, Couñago RM, Russo CA, Alves-Ferreira M (2009) Evolution of the B3 DNA binding superfamily: new insights into REM family gene diversification. PLoS One 4(6):e5791
Rorat T (2006) Plant dehydrins—tissue location, structure and function. Cell Mol Biol Lett 11:536–556
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Serrano R, Montesinos C (2003) Molecular bases of desiccation tolerance in plant cells and potential applications in food dehydration. Food Sci Technol Int 9:157–161
Shao HB, Liang ZS, Shao MA (2005) LEA proteins in higher plants: structure, function, gene expression and regulation. Colloid Surf B 45:131–135
Shiu SH, Bleecker AB (2003) Expansion of the receptor-like kinase/pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol 132:530–543. doi:10.1104/pp.103.021964
Singh S, Cornilescu CC, Tyler RC, Cornilescu G, Tonelli M, Lee MS, Markley JL (2005) Solution structure of a late embryogenesis abundant protein (LEA14) from Arabidopsis thaliana, a cellular stressrelated protein. Protein Sci 14(10):2601–2609
Söding J (2005) Protein homology detection by HMM-HMM comparison. Bioinformatics 21:951–960
Suo J, Liang X, Pu L, Zhang Y, Xue Y (2003) Identification of GhMYB109 encoding a R2R3 MYB transcription factor that expressed specifically in fiber initials and elongating fibers of cotton (Gossypium hirsutum L.). Biochim Biophys Acta Gene Struct Expr 1630(1):25–34
Suyama M, Torrents D, Bork P (2006) PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res 34:W609–W612. doi:10.1093/nar/gkl315
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729. doi:10.1093/molbev/mst197
Tang H, Bowers JE, Wang X et al (2008) Synteny and collinearity in plant genomes. Science 320:486–488. doi:10.1126/science.1153917
Tanurdzic M, Banks JA (2004) Sex-determining mechanisms in land plants. Plant Cell 16:S61–S71
Thompson JD, Gibson TJ, Plewniak F et al (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882. doi:10.1093/nar/25.24.4876
Tolleter D, Hincha DK, Macherel D (2010) A mitochondrial late embryogenesis abundant protein stabilizes model membranes in the dry state. Biochim Biophys Acta 1798:1926–1933
Tunnacliffe A, Hincha DK, Leprince O, Macherel D (2010) LEA Proteins: versatility of form and function. In: Lubzens E, Cerda J, Clark M (eds) Sleeping beauties—dormancy and resistance in harsh environments. Springer, Berlin, pp 91–108
Umezawa T, Fujita M, Fujita Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Engineering drought tolerance in plants: discovering and tailoring genes unlock the future. Curr Opin Biotechnol 17:113–122
Unver T, Budak H (2009) Conserved microRNAs and their targets in model grass species Brachypodium distachyon. Planta (ISI) 230(4):659–669
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78
Wang XS, Zhu HB, Jin GL, Liu HL, Wu WR, Zhu J (2007) Genome-scale identification and analysis of LEA genes in rice (Oryza sativa L.). Plant Sci 172(2):414–420
Wang L, Li X, Chen S, Liu G (2009) Enhanced drought tolerance in transgenic Leymus chinensis plants with constitutively expressed wheat TaLEA (3). Biotechnol Lett 31(2):313–319. doi:10.1007/s10529-008-9864-5
Weaver LM, Gan S, Quirino B, Amasino RM (1998) A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatment. Plant Mol Biol 37:455–469
Wen X, Niu T, Kong X (2014) In silico analysis of PHB gene family in maize. Plant Growth Regul 73(2):181–191
Wise MJ (2003) LEA ping to conclusions: a computational reanalysis of late embryogenesis abundant proteins and their possible roles. BMC Bioinform 4:52
Wolkers WF, McCready S, Brandt WF, Lindsey GG, Hoekstra FA (2001) Isolation and characterization of a D-7 LEA protein from pollen that stabilizes glasses in vitro. Biochim Biophys Acta 1544:196–206
Xoconostle Cázares B, Xiang Y, Ruiz-Medrano R, Wang HL, Monzer J, Yoo BC, McFarland KC, Lucas WJ (1999) Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science 283:94–98
Yang Z, Gu S, Wang X et al (2008) Molecular evolution of the cpp-like gene family in plants: insights from comparative genomics of Arabidopsis and rice. J Mol Evol 67:266–277. doi:10.1007/s00239-008-9143-z
Yu Y, Xia X, Yin W, Zhang H (2007) Comparative genomic analysis of CIPK gene family in Arabidopsis and Populus. Plant Growth Regul 52(2):101–110
Zhang Y (2005) miRU: an automated plant miRNA target prediction server. Nucleic Acids Res 33:W701–W704
Zhao P, Liu F, Ma M, Gong J, Wang Q, Jia P, Zheng G, Liu H (2011) Overexpression of AtLEA3-3 confers resistance to cold stress in Escherichia coli and provides enhanced osmotic stress tolerance and ABA sensitivity in Arabidopsis thaliana. Mol Biol 45(5):785–796. doi:10.1134/s0026893311050165
Zhou L, Liu Y, Liu Z, Kong D, Duan M, Luo L (2010) Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. J Exp Bot 61(15):4157–4168
Acknowledgments
This work was financially supported by The Scientific and Technological Research Council of Turkey (TUBITAK) with grant number 114O761.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Exon-intron organization of cucumber CsLEA genes. LEA gene family members in cucumber are classified according to Fig. 2 Clusters. Exons are showed as yellow boxes and introns are grey lines (JPEG 215 kb)
Fig. S2
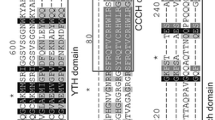
Presentation of motif clades for the CsLEA proteins. Fifteen different MEME motifs are distributed as different colors (JPEG 54 kb)
Fig. S3
Comparative physical mapping indicated high degree of orthologous relationships of LEA genes located on cucumber chromosomes with poplar, Arabidopsis and maize. Orthologous genes between cucumber and poplar, Arabidopsis and maize are presented by red lines (JPEG 322 kb)
Fig. S4
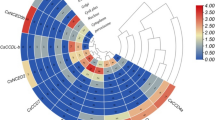
Heatmap distribution of CsLEA genes, which showing expression patterns of these gene family members in the 10 different tissues and organ systems of cucumber using transcriptome data. Expression values indicated as color gradient from no expression (green) to high expression (red) levels (TIFF 3108 kb)
Table S4
miRNA targets identified by psRNATarget (XLSX 14 kb)
Rights and permissions
About this article
Cite this article
Celik Altunoglu, Y., Baloglu, P., Yer, E.N. et al. Identification and expression analysis of LEA gene family members in cucumber genome. Plant Growth Regul 80, 225–241 (2016). https://doi.org/10.1007/s10725-016-0160-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-016-0160-4