Abstract
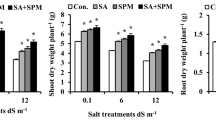
No information is available concerning effective microorganisms (EM) influence on the ionic and osmotic responses in plants grown in salty soils. Therefore, as a first approach, this study focuses on the contribution of EM to nutrient acquisition and compatible solutes accumulation in salt-stressed plants. It assesses some mechanisms underlying alleviation of salt toxicity by EM application, and also directs to establish a possible interrelationship between EM application as well as ionic and osmotic stresses tolerance in plants exposed to saline soils. Phaseolus vulgaris cv. Nebraska plants were grown under non-saline or saline conditions (2.5 and 5.0 dS m−1) with and without EM application. Salinity stress significantly decreased growth, productivity, membrane stability index, relative water content, concentrations of N, P, K+, Fe, Zn and Cu, and the ratios of K+/Na+, Ca2+/Na+ and Mg2+/Na+. However, EM application protected plants against the detrimental effect of salinity and significantly improved the above parameters. Concentrations of Ca+2, Mg+2, soluble sugars, free amino acids, proline and glycinebetaine were increased under saline conditions; moreover they further increased in salt-stressed plants treated with EM. Lipid peroxidation, hydrogen peroxide content, electrolyte leakage and Na+ level were increased in response to salinity and significantly decreased when stressed plants treated by EM. Reduction in Na uptake together with a concomitant increase in N, P, K, Ca, Mg, Fe, Zn and Cu absorption and a high compatible solutes accumulation may be an efficient mechanism used by EM-treated plants to gain tolerance against salinity stress.









Similar content being viewed by others
References
Abdel-Fattah GM, Asrar AA (2012) Arbuscular mycorrhizal fungal application to improve growth and tolerance of wheat (Triticum aestivum L.) plants grown in saline soil. Acta Physiol Plant 34:267–277
Allen SE (1989) Chemical analysis of ecological materials, 2nd edn. Blackwell, Oxford
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Bates LS, Aldren RP, Teare LD (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bayuelo-Jimenez JS, Jasso-Plata N, Ochoa I (2012) Growth and physiological responses of phaseolus species to salinity stress. Int J Agron 2012:1–13
Cottenie A, Verloo M, Kiekens L, Velghe G, Camerlynck R (1982) Chemical analysis of plants and soils. Laboratory of Analytical and Agrochemistry. State University, Ghent, pp 14–24
D’Souza MR, Devaraj VR (2010) Biochemical responses of Hyacinth bean (Lablab purpureus) to salinity stress. Acta Physiol Plant 32:341–353
Demidchik V, Davenport RJ, Tester M (2002) Nonselective cation channels in plants. Annu Rev Plant Biol 53:67–107
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307
Hayat S, Ali B, Hasan SA, Ahmad A (2007) Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ Exp Bot 60:33–41
Higa T (2000) What is EM technology? EM world J 1:1–6
Higa T (2004) Effective microorganisms—a new dimension for nature farming. In: Parr JF, Hornick SB, Simpson ME (eds) Proceedings of the 2nd international nature farming conference. U.S. Department of Agriculture; Washington, DC, USA, pp 20–22
Hodges MD, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Hu C, Qi Y (2013) Long-term effective microorganisms application promote growth and increase yields and nutrition of wheat in China. Eur J Agron 46:63–67
Irigoyen JJ, Emerich DW, Sanchez-Dıaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugar in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84:55–60
Ismail SM (2013) Influence of effective microorganisms and green manure on soil properties and productivity of pearl millet and alfalfa grown on sandy loam in Saudi Arabia. Afr J Microbiol Res 7:375–382
Jana S, Choudhari MA (1981) Glycolate metabolism of three submerged aquatic angiosperms during ageing. Aquat Bot 12:345–354
Javaid A, Bajwa R (2011) Field evaluation of effective microorganisms (EM) application for growth, nodulation, and nutrition of mung bean. Turk J Agric For 35:443–452
Kavi Kishor PB, Sangam S, Amrutha RN, Sri Laxmi P, Naidu KR, Rao KRSS, Rao S, Reddy KJ, Theriappan P, Sreenivasulu N (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88:424–438
Khedr AH, Abbas MA, Abdel Wahid AA, Quick WP, Abogadallah GM (2003) Proline induces the expression of salt-stress responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt-stress. J Exp Bot 54:2553–2562
Köhler B, Raschke K (2000) The delivery of salts to the xylem. Three types of anion conductance in the plasmalemma of the xylem parenchyma of roots of barley. Plant Physiol 122:243–254
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78:389–398
Miklas PN, Kelly JD, Beebe SE, Blair MW (2006) Common bean breeding for resistance against biotic and abiotic stresses: from classical to MAS breeding. Euphytica 147:105–131
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Moller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Moore S, Stein WH (1954) A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem 211:907–913
Munns R (1993) Physiological responses limiting plant growth in saline soils: some dogmas and hypotheses. Plant, Cell Environ 16:15–24
Ndona RK, Friedel JK, Spornberger A, Rinnofner T, Jezik K (2011) Effective micro-organisms (EM): an effective plant strengthening agent for tomatoes in protected cultivation. Biol Agric Hortic 27:189–204
Panda SK, Khan MH (2009) Growth, oxidative damage and antioxidant responses in greengram (Vigna radiata L.) under short-term salinity stress and its recovery. J Agron Crop Sci 195:442–454
Pareek-Singla SL, Grover A (1997) Salt responsive proteins/genes in crop plants. In: Jaiwal PK, Singh RP, Gulati A (eds) Strategies for improving salt tolerance in higher plants. Oxford and IBH Publishing Co, New Delhi, pp 365–391
Parida AK, Das AB (2005) Salt tolerance and salinity effect of plants: a review. Ecotoxicol Environ Saf 60:324–349
Parida AK, Das AB, Das P (2002) NaCl stress causes changes in photosynthetic pigments, proteins and other metabolic components in the leaves of a true mangrove, Bruguiera parviflora, in hydroponic cultures. J Plant Biol 45:28–36
Plaut Z, Grieve CM (1988) Photosynthesis of salt stressed maize as influenced by Ca:Na ratios in the nutrient solution. Plant Soil 105:283–286
Pregl F (1945) Quantitative organic micro analysis, 4th edn. A. Churchill Ltd., London
Rabie GH, Almadini AM (2005) Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr J Biotech 4:210–222
Ramoliya P, Patel H, Pandey AN (2004) Effect of salinization of soil on growth and macro- and micro-nutrient accumulation in seedlings of Salvadora persica (Salvadoraceae). For Ecol Manag 2002:181–193
Sairam RK (1994) Effects of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture stress conditions of two wheat varieties. Plant Growth Regul 14:173–181
Shiyab SM, Shatnawi MA, Shibli RA, Al Smeirat NG, Ayad J, Akash MW (2013) Growth, nutrient acquisition and physiological responses of hydroponic grown tomato to sodium chloride salt induced stress. J Plant Nutr 36:665–676
Snedecor GW, Cochran WG (1980) Statistical methods, 7th edn. Iowa State Univ. Press, Ames
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Talaat NB, Shawky BT (2013a) 24-Epibrassinolide alleviates salt-induced inhibition of productivity by increasing nutrients and compatible solutes accumulation and enhancing antioxidant system in wheat (Triticum aestivum L.). Acta Physiol Plant 35:729–740
Talaat NB, Shawky BT (2013b) Modulation of nutrient acquisition and polyamine pool in salt-stressed wheat (Triticum aestivum L.) plants inoculated with arbuscular mycorrhizal fungi. Acta Physiol Plant 35:2601–2610
Talaat NB, Shawky BT (2014a) Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ Exp Bot 98:20–31
Talaat NB, Shawky BT (2014b) Modulation of the ROS-scavenging system in salt-stressed wheat plants inoculated with arbuscular mycorrhizal fungi. J Plant Nutr Soil Sci 177:199–207
Yang X, Liang Z, Wen X, Lu C (2008) Genetic engineering of the biosynthesis of glycinebetaine leads to increased tolerance of photosynthesis to salt stress in transgenic tobacco plants. Plant Mol Biol 66:73–86
Yildirim E, Karlidag H, Turan M (2009) Mitigation of salt stress in strawberry by foliar K, Ca and Mg nutrient supply. Plant Soil Environ 55:213–221
Yokoi S, Quintero FJ, Cubero B, Ruiz MT, Bressan RA, Hasegawa PM, Pardo JM (2002) Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J 30:529–539
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talaat, N.B., Ghoniem, A.E., Abdelhamid, M.T. et al. Effective microorganisms improve growth performance, alter nutrients acquisition and induce compatible solutes accumulation in common bean (Phaseolus vulgaris L.) plants subjected to salinity stress. Plant Growth Regul 75, 281–295 (2015). https://doi.org/10.1007/s10725-014-9952-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-014-9952-6