Abstract
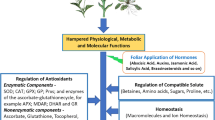
Due to the increasing demand for biofuel production, it is an important goal to optimize the seed productivity and quality of oilseed plants even in adverse conditions. Acting on signalling mechanisms might provide means to attain such goals. In this study, we were interested in the effect of a brassinosteroid hormone 24-epibrassinolide (24-EBR) on Brassica napus cultivated in salt stress condition. We show that salt stress leads to a 60 % decrease in seed production in B. napus. This is accompanied by a 50 % decrease in seed oil content. Treatment with 24-EBR had no effect on seed and oil productivity in control plants. However, it could rescue half of the seed production and all the oil production in salt-treated plants. The fatty acid composition of seed oil in B. napus was selectively affected by salt stress, 24-EBR or combined treatment. Besides these long-term actions of 24-EBR, we have also investigated its short-term actions in cell signalling. We did so by in vivo labelling of plantlets with fluorescently labelled phosphatidylcholine. A treatment of 2 h with 24-EBR was sufficient to induce a substantial increase in the content of diacylglycerol and phosphatidic acid, two lipid mediators. Non-specific phospholipases C and phospholipases D are involved in these increases. Therefore, brassinosteroid treatments appear as promising way to gain oil productivity when plants have to grow in unfavourable conditions such as salt stress. The link between long-term actions and short-term signalling of 24-EBR is discussed.





Similar content being viewed by others
References
Albrecht C, Boutrot F, Segonzac C, Schwessinger B, Gimenez-Ibanez S, Chinchilla D, Rathjen JP, de Vries SC, Zipfel C (2011) Brassinosteroids inhibit pathogen-associated molecular pattern–triggered immune signaling independent of the receptor kinase BAK1. PNAS 109:303–308
Arisz SA, van Wijk Rv, Roels W, Zhu J-K, Haring MA, Munnik T (2013) Rapid phosphatidic acid accumulation in response to low temperature stress in Arabidopsis is generated through diacylglycerol kinase. Front Plant Sci 4(1). doi:10.3389/fpls.2013.00001
Asghar HA, Zahir ZZ, Arshad MA, Khaliq AK (2002) Relationship between in vitro production of auxins by rhizobacteria and their growth-promoting activities in Brassica juncea L. Biol Fertil Soils 35(4):231–237
Atkinson NJ, Urwin PE (2012) The interaction of plant biotic and abiotic stresses: from genes to the field. J Exp Bot 63:3523–3543
Bates PD, Browse J (2012) The significance of different diacylglycerol synthesis pathways on plant oil composition and bioengineering. Front Plant Sci 3 (147). doi:10.3389/fpls.2012.00147
Beisson F, Koo AJK, Ruuska S, Schwender J, Pollard M, Thelen JJ, Paddock T, Salas JJ, Savage L, Milcamps A, Mhaske VB, Cho Y, Ohlrogge JB (2003) Arabidopsis genes involved in acyl lipid metabolism. A 2003 census of the candidates, a study of the distribution of expressed sequence tags in organs, and a web-based database. Plant Physiol 132(2):681–697
Chapman KD, Ohlrogge JB (2012) Compartmentation of triacylglycerol accumulation in plants. J Biol Chem 287(4):2288–2294
Chaytor JP (1987) Analysis of fatty acids in lipids by high performance liquid chromatography (HPLC). Food Chem 23(1):19–27
Clouse SD (2011) Brassinosteroid signal transduction: from receptor kinase activation to transcriptional networks regulating plant development. Plant Cell Online 23(4):1219–1230
Divi U, Rahman T, Krishna P (2010) Brassinosteroid-mediated stress tolerance in arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol 10(1):1–14
Djafi N, Vergnolle C, Cantrel C, Wietrzynski W, Delage E, Cochet F, Puyaubert J, Soubigou-Taconnat L, Gey D, Collin S, Balzergue S, Zachowski A, Ruelland E (2013) The Arabidopsis DREB2 genetic pathway is constitutively repressed by basal phosphoinositide-dependent phospholipase C coupled to diacylglycerol kinase in Arabidopsis thaliana. Front Plant Sci 4. doi:10.3389/fpls.2013.00307
Evers D, Legay S, Lamoureux D, Hausman J, Hoffmann L, Renaut J (2012) Towards a synthetic view of potato cold and salt stress response by transcriptomic and proteomic analyses. Plant Mol Biol 78(4):503–514
Francois LE (1994) Growth, seed yield, and oil content of canola grown under saline conditions. Agron J 86(2):233–237
Furukawa-Stoffer TL, Byers SD, Hodges DM, Laroche A, Weselake RJ (1998) Identification of N-ethylmaleimide-sensitive and -insensitive phosphatidate phosphatase activity in microspore-derived cultures of oilseed rape. Plant Sci 131(2):139–147
Goda H, Shimada Y, Asami T, Fujioka S, Yoshida S (2002) Microarray analysis of brassinosteroid-regulated genes in Arabidopsis. Plant Physiol 130(3):1319–1334
Grindstaff KK, Fielding LA, Brodl MR (1996) Effect of gibberellin and heat shock on the lipid composition of endoplasmic reticulum in barley aleurone layers. Plant Physiol 110(2):571–581
Hong Y, Pan X, Welti R, Wang X (2008) Phospholipase Dα3 is involved in the hyperosmotic response in Arabidopsis. The Plant Cell Online 20(3):803–816
Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P (2008) Genevestigator V3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinform 2008:420747
Hua W, Li R-J, Zhan G-M, Liu J, Li J, Wang X-F, Liu G-H, Wang H-Z (2012) Maternal control of seed oil content in Brassica napus: the role of silique wall photosynthesis. Plant J 69(3):432–444
Janda M, Planchais S, Djafi N, Martinec J, Burketova L, Valentova O, Zachowski A, Ruelland E (2013) Phosphoglycerolipids are master players in plant hormone signal transduction. Plant Cell Rep. doi:10.1007/s00299-013-1399-0
Janeczko A, Biesaga K, Cielniak J, Dziurka M (2009) 24-Epibrassinolide modifies seed composition in soybean, oilseed rape and wheat. Seed Sci Technol 37(3):625–639
Kagale S, Divi U, Krochko J, Keller W, Krishna P (2007) Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 225(2):353–364
Khripach V (1990) Synthesis of brassinosteroids. Pure Appl Chem 62(7):1319–1324
Kim T-W, Wang Z-Y (2010) Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu Rev Plant Biol 61(1):681–704
Kim T-W, Michniewicz M, Bergmann DC, Wang Z-Y (2012) Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature 482(7385):419–422
Kocourková D, Krčková Z, Pejchar P, Veselková Š, Valentová O, Wimalasekera R, Scherer GFE, Martinec J (2011) The phosphatidylcholine-hydrolysing phospholipase C NPC4 plays a role in response of Arabidopsis roots to salt stress. J Exp Bot 62(11):3753–3763
Kravets VS, Kolesnikov YS, Kretynin SV, Getman IA, Romanov GA (2010) Rapid activation of specific phospholipase(s) D by cytokinin in Amaranthus assay system. Physiol Plant 138(3):249–255
Krinke O, Flemr M, Vergnolle C, Collin S, Renou J-P, Taconnat L, Yu A, Burketová L, Valentová O, Zachowski A, Ruelland E (2009) Phospholipase D activation is an early component of the salicylic acid signalling pathway in Arabidopsis cell suspensions. Plant Physiol 150(1):424–436
Lee J, Welti R, Schapaugh WT, Trick HN (2011) Phospholipid and triacylglycerol profiles modified by PLD suppression in soybean seed. Plant Biotechnol J 9(3):359–372
Lee J, Welti R, Roth M, Schapaugh WT, Li J, Trick HN (2012) Enhanced seed viability and lipid compositional changes during natural ageing by suppressing phospholipase Dα in soybean seed. Plant Biotechnol J 10(2):164–173
Li M, Welti R, Wang X (2006) Quantitative profiling of Arabidopsis polar glycerolipids in response to phosphorus starvation. Roles of phospholipases Dζ1 and Dζ2 in phosphatidylcholine hydrolysis and digalactosyldiacylglycerol accumulation in phosphorus-starved plants. Plant Physiol 142(2):750–761
Li B, Zhang C, Cao B, Qin G, Wang W, Tian S (2012) Brassinolide enhances cold stress tolerance of fruit by regulating plasma membrane proteins and lipids. Amino Acids 43(6):2469–2480
Liu W, Hildebrand DF, Collins GB (1995) Auxin-regulated changes of fatty acid content and composition in soybean zygotic embryo cotyledons. Plant Sci 106(1):31–42
Maatta S, Scheu B, Roth MR, Tamura P, Li M, Williams TD, Wang X, Welti R (2012) Levels of Arabidopsis thaliana leaf phosphatidic acids, phosphatidylserines, and most trienoate-containing polar lipid molecular species increase during the dark period of the diurnal cycle. Front Plant Sci 3 (49). doi:10.3389/fpls.2012.00049
McLoughlin F, Arisz SA, Dekker HL, Kramer G, de Koster CG, Haring MA, Munnik T, Testerink C (2013) Identification of novel candidate phosphatidic acid-binding proteins involved in the salt-stress response of Arabidopsis thaliana roots. Biochem J 450(3):573–581
Ohlrogge JB, Jaworski JG (1997) Regulation of fatty acid synthesis. Annu Rev Plant Physiol Plant Mol Biol 48(1):109–136
Pejchar P, Potocký M, Novotná Z, Veselková Š, Kocourková D, Valentová O, Schwarzerová K, Martinec J (2010) Aluminium ions inhibit the formation of diacylglycerol generated by phosphatidylcholine-hydrolysing phospholipase C in tobacco cells. New Phytol 188(1):150–160
Peng Y, Zhang J, Cao G, Xie Y, Liu X, Lu M, Wang G (2010) Overexpression of a PLDα1 gene from Setaria italica enhances the sensitivity of Arabidopsis to abscisic acid and improves its drought tolerance. Plant Cell Rep 29(7):793–802
Pleskot R, Pejchar P, Bezvoda R, Lichtscheidl IK, Wolters-Arts M, Marc J, Žárský V, Potocký M (2012) Turnover of phosphatidic acid through distinct signalling pathways affects multiple aspects of tobacco pollen tube tip growth. Front Plant Sci 3 (54). doi:10.3389/fpls.2012.00054
Pokotylo I, Kretinin S, Kravets V (2012) Role of phospholipase D in metabolic reactions of transgenic tobacco cax1 cells under the influence of salt stress. Cytol Genetics 46(3):131–135
Pokotylo I, Pejchar P, Potocký M, Kocourková D, Krčková Z, Ruelland E, Kravets V, Martinec J (2013) The plant non-specific phospholipase C gene family. Novel competitors in lipid signalling. Prog Lipid Res 52(1):62–79
Rainteau D, Humbert L, Delage E, Vergnolle C, Cantrel C, Maubert M-A, Lanfranchi S, Maldiney R, Collin S, Wolf C, Zachowski A, Ruelland E (2012) Acyl chains of phospholipase D transphosphatidylation products in Arabidopsis cells: a study using multiple reactions monitoring mass spectrometry. PLoS ONE 7(7):e41985
Román Á, Andreu V, Hernández ML, Lagunas B, Picorel R, Martínez-Rivas JM, Alfonso M (2012) Contribution of the different omega-3 fatty acid desaturase genes to the cold response in soybean. J Exp Bot 63(13):4973–4982
Ruiz-López N, Sayanova O, Napier JA, Haslam RP (2012) Metabolic engineering of the omega-3 long chain polyunsaturated fatty acid biosynthetic pathway into transgenic plants. J Exp Bot 63(7):2397–2410
Siaut M, Cuine S, Cagnon C, Fessler B, Nguyen M, Carrier P, Beyly A, Beisson F, Triantaphylides C, Li-Beisson Y, Peltier G (2011) Oil accumulation in the model green alga Chlamydomonas reinhardtii: characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol 11(1):7
Sudriá C, Palazón J, Cusidó R, Bonfill M, Piñol MT, Morales C (2001) Effect of benzyladenine and indolebutyric acid on ultrastructure, glands formation, and essential oil accumulation in Lavandula dentata plantlets. Biol Plant 44(1):1–6
Tan H, Yang X, Zhang F, Zheng X, Qu C, Mu J, Fu F, Li J, Guan R, Zhang H, Wang G, Zuo J (2011) Enhanced seed oil production in canola by conditional expression of Brassica napus LEAFY COTYLEDON1 and LEC1-LIKE in developing seeds. Plant Physiol 156(3):1577–1588
Tang M, Guschina IA, O’Hara P, Slabas AR, Quant PA, Fawcett T, Harwood JL (2012) Metabolic control analysis of developing oilseed rape (Brassica napus cv Westar) embryos shows that lipid assembly exerts significant control over oil accumulation. New Phytol 196(2):414–426
Voelker T, Kinney AJ (2001) Variations in the biosynthesis of seed-storage lipids. Annu Rev Plant Physiol Plant Mol Biol 52(1):335–361
Wang G, Ryu S, Wang X (2012) Plant phospholipases: an overview. Lipases and phospholipases. In: Sandoval G (ed), vol 861. Methods in molecular biology. Humana Press, pp 123–137
Wimalasekera R, Pejchar P, Holk A, Martinec J, Scherer GFE (2010) Plant phosphatidylcholine-hydrolyzing phospholipases C NPC3 and NPC4 with roles in root development and brassinolide signaling in Arabidopsis thaliana. Mol Plant 3(3):610–625
Xia X-J, Huang L-F, Zhou Y-H, Mao W-H, Shi K, Wu J-X, Asami T, Chen Z, Yu J-Q (2009) Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 230(6):1185–1196
yoon Jeong S, Park C, Kim M-K, Jun Nam S, Hong J, Kim S-K (2012) Effect of lysophosphatidylethanolamine and brassinosteroids on development of Arabidopsis roots. J Plant Biol 55(2):178–184
Zhang Z, Ramirez J, Reboutier D, Brault M, Trouverie J, Pennarun A-M, Amiar Z, Biligui B, Galagovsky L, Rona J-P (2005) Brassinosteroids regulate plasma membrane anion channels in addition to proton pumps during expansion of Arabidopsis thaliana cells. Plant Cell Physiol 46(9):1494–1504
Zhang T, Song Y, Liu Y, Guo X, Zhu C, Wen F (2008) Overexpression of phospholipase Dα gene enhances drought and salt tolerance of Populus tomentosa. Chin Sci Bull 53(23):3656–3665
Zhang A, Zhang J, Ye N, Cao J, Tan M, Zhang J, Jiang M (2010) ZmMPK5 is required for the NADPH oxidase-mediated self-propagation of apoplastic H2O2 in brassinosteroid-induced antioxidant defence in leaves of maize. J Exp Bot 61(15):4399–4411
Zhang J, Liu H, Sun J, Li B, Zhu Q, Chen S, Zhang H (2012) Arabidopsis fatty acid desaturase FAD2 is required for salt tolerance during seed germination and early seedling growth. PLoS ONE 7(1):e30355
Acknowledgments
We thank Institute of Agriculture NAAS for providing seeds of B. napus cv. Magnat plants. We are also grateful to V.P. Grahov for the help with HPLC analysis. This work was supported by NAS of Ukraine (Grants No. 18–12 and No. 9.1–12).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pokotylo, I.V., Kretynin, S.V., Khripach, V.A. et al. Influence of 24-epibrassinolide on lipid signalling and metabolism in Brassica napus . Plant Growth Regul 73, 9–17 (2014). https://doi.org/10.1007/s10725-013-9863-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-013-9863-y