Abstract
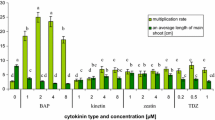
As preliminary research to develop a clonal in vitro propagation system for saffron, we have evaluated the morphogenic response of intercalary meristems present in shoots from sprouting saffron corms when cultured in media with different cytokinins. The best results in terms of shoot production and quality were achieved in the medium supplemented with 5 mg/l of benzylaminopurine (BAP). During the multiplication of the meristems, several changes in the activities of the antioxidant enzymatic system were detected. Catalase (CAT) activity was reduced in the presence of the assayed cytokinins, whereas peroxidase (POX) activity was stimulated with all the cytokinins assayed. When TDZ (thidizuron) was used, a slight decrease in superoxide dismutase (SOD) activity occurred, whereas BAP and 2iP (2-isopentenyl adenine) treatments produced a significant increase in SOD activity. The ascorbate–glutathione cycle enzymes showed a similar pattern in response to the addition of cytokinins. These results suggest a possible use of antioxidant enzyme activities as a parameter that could be use to evaluate the efficiency of micropropagation protocols for saffron and even in other species.





Similar content being viewed by others
Abbreviations
- 2iP:
-
2-isopentyladenin
- APX:
-
Ascorbate peroxidase (EC 1.11.1.1)
- BAP:
-
6-benzylaminopurine
- CAT:
-
Catalase (EC 1.11.1.6)
- DHAR:
-
Dehydroascorbate reductase (EC 1.8.5.1)
- GR:
-
Glutathione reductase (EC 1.6.4.2)
- MDHAR:
-
Monodehydroascorbate reductase (EC 1.6.5.4)
- POX:
-
Peroxidases (EC 1.11.1.7)
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase (EC 1.15.1.1)
- TDZ:
-
Thidiazuron
References
Aebi H (1984) Catalase in vitro. In: Lester P (ed) Methods in enzymology oxygen radicals in biological systems. Academic Press, London, pp 121–126
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Ann Rev Plant Biol 55:373–399
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Ann Rev Plant Physiol Plant Mol Biol 50:601–639
Barba-Espin G, Diaz-Vivancos P, Clemente-Moreno MJ, Albacete A, Faize L, Faize M, Pérez-Alfocea F, Hernández JA (2010) Interplay between hydrogen peroxide and plant hormones during germination and the early growth of pea seedlings. Plant Cell Environ 33:981–994
Batkova P, Pospisilova J, Synkova H (2008) Production of reactive oxygen species and development of antioxidative ayatems during in vitro growth and ex vitro transfer. Biol Plant 52:413–422
Benson EE (2000) Do free radicals have a role in plant tissue culture recalcitrance? In Vitro Cell Dev Biol-Plant 36:163–170
Benson EE, Lynch PT, Jones J (1992) Variation in free-radical damage in rice cell-suspensions with different embryogenic potentials. Planta 188:296–305
Blázquez S, Olmos E, Hernandez JA, Fernandez-Garcia N, Fernandez JA, Piqueras A (2009) Somatic embryogenesis in saffron (Crocus sativus L) histological differentiation and implication of some components of the antioxidant enzymatic system. Plant Cell Tissue Organ Culture 97:49–57
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cakmak I, Strbac D, Marschner H (1993) Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J Exp Bot 44:127–132
Chahota RK, Dhiman KC, Rana SS, Singh M (2003) Efficacy of different propagating methods for higher daughter corm production in saffron (Crocus sativus L). Indian Perfumer 47:155–158
Chakrabarty D, Park SY, Ali MB, Shin KS, Paek KY (2006) Hyperhydricity in apple: ultrastructural and physiological aspects. Tree Physiol 26:377–388
Chen J, Ziv M (2001) The effect of ancymidol on hyperhydricity, regeneration, starch and antioxidant enzymatic activities in liquid-cultured Narcissus. Plant Cell Rep 20:22–27
Decker EL, Frank W, Sarnighausen E, Reski R (2006) Moss systems biology en route: phytohormones in physcomitrella development. Plant Biol 8:397–400
Del Rio LA, Pastori GM, Palma JM, Sandalio LM, Sevilla F, Corpas FJ, Jiménez A, Lopez-Huertas E, Hernandez JA (1998) The activated oxygen role of peroxisomes in senescente. Plant Physiol 116:1195–1200
Diaz-Vivancos P, Barba-Espin G, Clemente-Moreno MJ, Hernandez JA (2010) Characterization of the antioxidant system during the vegetative development of pea plants. Biol Plant 54:76–82
Foyer CH, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28:1056–1071
Gupta D (2010) Role of free radicals and antioxidants in in vitro morphogenesis. In: Gupta D (ed) Reactive oxygen species and antioxidants in higher plants. CRC Press, pp 230–247
Gupta SD, Datta S (2003) Antioxidant enzyme activities during in vitro morphogenesis of gladiolus and the effect of application of antioxidants on plant regeneration. Biol Plant 47:179–183
Hernandez JA, Jiménez A, Mullineaux PM, Sevilla F (2000) Tolerance of pea (Pisum sativum L) to long-term salt stress is associated with induction of antioxidant defenses. Plant Cell Environ 23:853–862
Hernandez JA, Ferrer MA, Jimenez A, Ros-Barcelo A, Sevilla F (2001) Antioxidant systems and O2 −/H2O2 production in the apoplast of Pisum sativum L leaves: its relation with NaCl-induced necrotic lesions in minor veins. Plant Physiol 127:817–831
Hernandez JA, Escoba C, Creissen G, Mullineaux PM (2004) Role of hydrogen peroxide and the redox state of ascorbate in the induction of antioxidant enzymes in pea leaves under excess light stress. Func Plant Biol 31:359–368
Imin N, Nizamidin M, Daniher D, Nolan KE, Rose RJ, Rolfe BG (2005) Proteomic analysis of somatic embryogenesis in medicago truncata explant cultures grown under 6-benzylaminopurine and 1-naphthaleneacetic acid treatments. Plant Physiol 137:1250–1260
Jimenez A, Hernandez JA, del Rio LA, Sevilla F (1997) Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 114:275–284
Jirage D, Ravishankar G, Suvarnlatha G, Venkataraman L (1994) Production of polyamines during sprouting and growth of saffron (Crocus sativus L) corms. Plant Growth Regul 13:69–72
Kataeva NV, Alexandrova IG, Butenko RG, Dragavtceva EV (1991) Effect of applied and internal hormones on vitrification and apical necrosis of different plants cultured in vitro. Plant Cell Tissue Organ Cul 27:149–154
Kawaoka A, Matsunaga E, Endo S, Kondo S, Yoshida K, Shinmyo A, Ebinuma H (2003) Ectopic expression of a horseradish peroxidase enhances growth rate and increases oxidative stress resistance in hybrid aspen. Plant Physiol 132:1177–1185
Kwak JM, Nguyen V, Schroeder JI (2006) The role of reactive oxygen species in hormonal responses. Plant Physiol 141:323–329
Lai Q-X, Bao Z-Y, Zhu Z-J, Qian Q-Q, Mao B-Z (2007) Effect of osmotic stress on antioxidant enzymes activities in leaf discs of PSAG12-IPT modified gerbera. J Zhejiang Univ Sci B 8:458–464
Majourhat K, Fernandez JA, Martinez-Gomez P, Piqueras A (2007) Enhanced plantlet regeneration from cultured meristems in sprouting buds of saffron corms. Acta Hortic 739:275–278
Mathew B (1982) The Crocus, a revision of the genus Crocus (Iridaceae). BT Batsford, London
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymic function for erythrocuprein. J Biol Chem 244:6049–6055
Molassiotis AN, Dimassi K, Diamantidis G, Therios I (2004) Changes in peroxidases and catalase activity during in vitro rooting. Biol Plant 48:1–5
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Negbi M, Dagan B, Dror A, Basker D (1989) Growth, flowering, vegetative reproduction, and dormancy in the saffron crocus (Crocus sativus L). Israel J Botany 38:95–113
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Ann Rev Plant Physiol Plant Mol Biol 49:249–279
Obert B, Benson EE, Millam S, Pretovb A, Bremner DH (2005) Moderation of morphogenetic and oxidative stress responses in flax in vitro cultures by hydroxynonenal and desferrioxamine. J Plant Physiol 162:537–547
Olmos E, Piqueras A, Martinez-Solano JR, Hellin E (1997) The subcellular localization of peroxidase and the implication of oxidative stress in hyperhydrated leaves of regenerated carnation shoots. Plant Sci 130:97–105
Pasternak TP, Potters G, Caubergs R, Jansen MAK (2005) Complementary interactions between oxidative stress and auxins control plant growth responses at plant, organ and cellular level. J Exp Bot 58:1991–2001
Piqueras A, Fernandez JA (2004) Phase change from dormancy to continuous shoot proliferation in cultured meristems of saffron corms. In Vitro Cell Dev Biol-Animal 40:74A
Piqueras A, Han BH, Van Huylenbroeck JM, Debergh PC (1998) Effect of different environmental conditions in vitro on sucrose metabolism and antioxidant enzymatic activities in cultured shoots of Nicotiana. Plant Growth Regul 25:5–10
Piqueras A, Han BH, Escribano J, Rubio C, Hellin E, Fernandez JA (1999) Development of cormogenic nodules and microcorms by tissue culture, a new tool for the multiplication and genetic improvement of saffron. Agronomie 19:603–610
Plessner O, Ziv M, Negbi M (1990) In vitro corm production in the saffron crocus (Crocus sativus L). Plant Cell Tissue Organ Culture 20:89–94
Pomar F, Caballero N, Pedreño MA, Ros Barceló A (2002) H2O2 generation during the auto-oxidation of coniferyl alcohol drives the oxidase activity of a highly conserved class III peroxidase involved in lignin biosynthesis. FEBS Lett 529:198–202
Quorin M, Lepoivre P (1977) Etude de milieux adaptes aux cultures in vitro de Prunus. Acta Hort 78:437–442
Romano CP, Hein MB, Klee HJ (1991) Inactivation of auxin in tobacco transformed with the indolacetic acid-lysine synthetase gene of Pseudomonas savastoni. Genes Dev 5:446–538
Ros Barcelo A (1998) The generation of H2O2 in the xylem of Zinnia elegans is mediated by an NADPH-oxidase-like enzyme. Planta 207:207–216
Ros Barceló A, Gómez-Ros LV, Ferrer MA, Hernández JA (2006) The apoplastic antioxidant enzymatic system in the wood-forming tissues of trees. Trees 20:145–156
Saher S, Piqueras A, Hellin E, Olmos E (2004) Hyperhydricity in micropropagated carnation shoots: the role of oxidative stress. Physiol Plant 120:152–161
Stephens JM (2003) Saffron-Crocus sativus L IFAS extension. Horticultural Sheets 661:2p
Synkova H, Semoradova S, Schnablova R, Witters E, Husak M, Valcke R (2006) Cytokinin-induced activity of antioxidant enzymes in transgenic Pssu-ipt tobacco during plant ontogeny. Biol Plant 50:31–41
Tang W, Newton RJ (2005) Peroxidase and catalase activities are involved in direct adventitious shoot formation induced by thidiazuron in eastern white pine (Pinus strobus L) zygotic embryos. Plant Physiol Biochem 43:760–769
Tang W, Harris LC, Outhavong V, Newton RJ (2004) Antioxidants enhance in vitro plant regeneration by inhibiting the accumulation of peroxidase in Virginia pine (Pinus virginiana Mill). Plant Cell Rep 22:871–877
Vatankhah E, Niknam V, Ebrahimzadeh H (2010) Activity of antioxidant enzyme during in vitro organogenesis in Crocus sativus. Biologia Plantarum 54(3):509–514
Veljovic-Jovanovic SD, Pignocchi C, Noctor G, Foyer CH (2001) Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant. Plant Physiol 127:426–435
Werner T, Schmülling T (2009) Citokinin action in plant development. Current Opinion Plant Biol 12:527–538
Yeung EC, Law SK (1987) Serial sectioning techniques for a modified Lkb historesin. Stain Technol 62:147–153
Zavaleta-Mancera HA, Lopez-Delgado H, Loza-Tavera H, Mora-Herrera M, Trevilla-Garcia C, Vargas-Suarez M, Ougham H (2007) Cytokinin promotes catalase and ascorbate peroxidase activities and preserves the chloroplast integrity during dark-senescence. J Plant Physiol 164:1572–1582
Acknowledgments
This research has been mainly supported by the projects PII1I09-0029-9367 (Consejería de Educación y Ciencia, JCCM, Spain), RF2004-0032-C03, PET2007-014-C05-03 (Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria, MEC, Spain), and AGRI GEN RES 018 Action ‘‘Genetic Resources of Saffron and Allies’’ (CROCUSBANK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Díaz-Vivancos, P., Majourhat, K., Fernández, J.A. et al. Study of the antioxidant enzymatic system during shoot development from cultured intercalar meristems of saffron. Plant Growth Regul 65, 119–126 (2011). https://doi.org/10.1007/s10725-011-9581-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-011-9581-2