Abstract
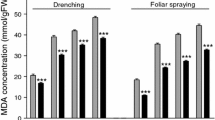
Detached leaves of tomato (Lycopersicon esculentum Mill.) experienced photoinhibition associated with sharp reductions in net photosynthetic rate (Pn), quantum efficiency of PSII (ΦPSII) and photochemical quenching (qP) even though they were exposed to mild light intensity (400 μmol m−2 s−1 PPFD) at 28°C. Photoinhibition and the reduction in Pn, ΦPSII and qP, however, were significantly alleviated by 1 mg l−1 ABA, 0.1 mg l−1 N-(2-chloro-4-pyridyl)-N′-phenylurea (CPPU) and 0.01 mg l−1 24-epibrassinolide (EBR). Higher concentrations, however, reduced the effects or even exacerbated the occurrence of photoinhibition. Superoxide dismutase and ascorbate peroxidase activity in leaves increased with the increases in ABA concentration within 1–100 mg l−1, CPPU concentration within 0.1–10 mg l−1 and EBR concentration within 0.01–1.0 mg l−1. Catalase and guaiacol peroxidase activity also increased with the increase in EBR concentration but CPPU and ABA treatments at higher concentrations caused a decrease. Malondialdehyde (MDA) content decreased with the increase in CPPU concentration. ABA and EBR, however, decreased MDA concentration only at 1 and 0.01 mg l−1, respectively. In conclusion, detached leaves had increased sensitivity to PSII photoinhibition. Photoinhibition-induced decrease in photosynthesis, however, was significantly alleviated by EBR, CPPU and ABA at a proper concentration.




Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- APX:
-
Ascorbate peroxidase
- BRs:
-
Brassinosteroids
- CAT:
-
Catalase
- CPPU:
-
N-(2-Chloro-4-pyridyl)-N′-phenylurea
- CTK:
-
Cytokinin
- EBR:
-
Brassinosteroids
- Fo, Fm, Fv:
-
Minimal, maximal and variable fluorescence yields
- Fm′, Fv′, Fs′:
-
Maximal, variable and steady state fluorescence yield in a light adapted state
- Fv/Fm:
-
The maximal photochemical efficiency of PSII
- Fv′/Fm′:
-
The efficiency of excitation energy capture by open PSII reaction centre
- G-POD:
-
Guaiacol peroxidase
- MDA:
-
Malondialdehyde
- NPQ:
-
Non photochemical quenching
- Pn:
-
Net photosynthetic rate
- PPFD:
-
Photosynthetic photon flux density
- ΦPSII :
-
Relative quantum efficiency of PSII photochemistry
- qP:
-
Photochemical quenching
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Ahmad P, Jaleel CA, Azooz MM, Nabi G (2009) Generation of ROS and non-enzymatic antioxidants during abiotic stress in plants. Bot Res Int 2(1):11–20
Allen DJ, Ort DR (2001) Impact of chilling temperatures on photosynthesis in warm climate plants. Trends Plant Sci 6:36–42
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol 55:373–399
Asada K, Takahashi M (1987) Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osmond CB, Amtzen CJ (eds) Photoinhibition. Elsevier, Amsterdam, pp 227–228
Baker NR, Nogues S, Allen DJ (1997) Photosynthesis and photoinhibition. In: Lumsden PJ (ed) Plants and UV-B; response to environmental change. Cambridge University Press, Cambridge, pp 95–111
Bellaire BA, Carmody J, Braud J, Gosset DR, Banks SW, Lucas MC, Fowler TE (2000) Involvement of abscisic acid-dependent and independent pathways in the upregulation of antioxidant enzyme activity during NaCl stress in cotton callus tissue. Free Radic Res 33:531–545
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein—dye binding. Anal Biochem 72:248–254
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227
Carmi A, Koller D (1978) Effects of the root on the rate of photosynthesis in primary leaves of bean (Phaseolus vulgaris L.). Photosynthetica 12:178–184
Chernyadev II (2009) The protective action of cytokinins on the photosynthetic machinery and productivity of plants under stress (review). App Biochem Microbiol 45(4):351–362
Clouse SD, Sasse JM (1998) Brassinosteroids: essential substances of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol 49:427–451
Dhaubhadel S, Chaundhary S, Dobinson KF, Krishna P (1999) Treatment with 24-epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napus and tomato seedlings. Plant Mol Biol 40:333–342
Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119:355–364
Genty B, Briatais JM, Baker NR (1989) The relationships between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Giannopolitis CN, Ries SK (1977) Superoxide dismutase1. Occurrence in higher plants. Plant Physiol 59:309–414
Guan L, Zhao J, Scandalios JG (2000) cis–Elements and trans-factors that regulate expression of the maize cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J 22:87–95
Hare PD, Van Staden J (1997) The molecular basis of cytokinin action. Plant Growth Regul 23:41–78
Horton P, Ruban AV, Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47:655–684
Hu WH, Zhou YH, Du YS, Xia XJ, Yu JQ (2006) Differential response of photosynthesis in greenhouse- and field-ecotypes of tomato to long-term chilling under low light. J Plant Physiol 163:1238–1246
Jia HS, Lu CM (2003) Effects of abscisic acid on photoinhibition in maize plants. Plant Sci 165:1403–1410
Jiang M, Zhang J (2001) Effects of abscisic acid on active oxygen species, antioxidative defense system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42:1265–1273
Larkindale J, Huang B (2004) Thermotolerance and antioxidant systems in Agrostis stolonifera: Involvement of salicyclic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. J Plant Physiol 161:405–413
Lawlor DW (2009) Musings about the effects of environment on photosynthesis. Ann Bot 103:543–549
Leakey L, Nada K, Tachibana S (2003) ABA-induced increase in thermotolerance of photosynthetic electron transport of thylakoids in leaves of cucumber (Cucumis sativus L.). J Japan Soc Hortic Sci 72(1):29–35
Liu X, Huang B, Banowetz G (2002) Cytokinin effects on creeping bentgrass response to heat stress: 1. Shoot and root growth. Crop Sci 42:457–465
Liu Y, Zhao Z, Si J, Di C, Han J, An L (2009) Brassinosteriods alleviate chilling-induced oxidative damage by enhancing antioxidant defense system in suspension cultured cells of Chorispora bungeana. Plant Growth Regul 59(3):207–214
Mazzora LM, Núñez M, Hechararria M, Coll F, Sanchez-Blanco MJ (2002) Influence of brassinosteroids on antioxidant enzyme activity in tomato under different temperatures. Biol Plant 45:593–596
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Montoya T, Nomura T, Yokota T, Farrar K, Harrison K, Jones JGD, Kaneta T, Kamiya W, Szekeres M, Bishop GR (2005) Patterns of dwarf expression and brassinosteroid accumulation in tomato reveal the importance of brassinosteroid synthesis during fruit development. Plant J 42:262–269
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:676–690
Noctor G (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Núñez M, Mazzafera P, Mazzora LM, Sigueira WJ, Zullo MAT (2003) Influence of a brassinosteroid analogue on antioxidant enzymes in rice grown in culture medium with NaCl. Biol Plant 47:67–70
Ogweno JO, Song XS, Shi K, Hu WH, Mao WH, Zhou YH, Yu JQ, Nogués S (2008) Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J Plant Growth Regul 27:49–57
Ort DR, Baker NR (2002) A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr Opin Plant Biol 5:193–198
Pfannschmidt T (2003) Chloroplast redox signals: how photosynthesis controls its own genes. Trends Plant Sci 8:33–41
Pogany M, Elstner EF, Barna B (2003) Cytokinin gene introduction confers tobacco necrosis virus resistance and higher antioxidant levels in tobacco. Free Radical Res 37:15–16
Sarvikas P, Tyystjarvi T, Tyystjarvi E (2010) Kinetics of prolonged photoinhibition revisited: photoinhibited photosystem II centres do not protect the active ones against loss of oxygen evolution. Photosynthesis Res. doi:10.1007/s11120-009-9496-1
Sasse JM (2003) Physiological actions of brassinosteroids: an update. J Plant Growth Regul 22:276–288
Sharma PK, Sankhalkar S, Fernandes Y (2002) Possible function of ABA in protection against photodamage by stimulating xanthophyll cycle in sorghum seedlings. Curr Sci 82(2):167–171
Takahashi S, Milward SE, Fan DY, Chow WS, Badger MR (2009) How does cyclic electron flow alleviate photoinhibition in Arabidopsis? Plant Physiol 149:1560–1567
Tholen D, Pons TL, Voesenek LACJ, Poorter H (2007) Ethylene insensitivity results in down-regulation of rubisco expression and photosynthetic capacity in tobacco. Plant Physiol 144:1305–1315
Udomprasert N, Li PH, Davis W, Markhart AH III (1995) Root cytokinin leveling relation to heat tolerance of Phaseolus acutifolius and Phaseolus vulgaris. Crop Sci 35:486–490
Ushimaru T, Kanazawa S, Sano S, Koshtoa T (2000) Changes in antioxidative enzymes in cucumber cotyledons during natural senescence: comparison with those during dark induced senescence. Physiol Plant 109:211–226
Von Kooten O, Snel J (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
Xin Z, Li PH (1992) Abscisic acid—induced chilling tolerance in maize in suspension- cultured cells. Plant Physiol 99:707–711
Xiong L, Zhu JK (2003) Regulation of abscisic acid biosynthesis. Plant Physiol 133:29–36
Yordanov I, Tsonev T, Goltsev V, Merakchiiska-Nikolova M, Georgieva K (1997) Gas exchange and chlorophyll fluorescence during water and high temperature stress and recovery. Probable protective effect of carbamide cytokinin 4-PU30. Photosynthetica 33:423–431
Yu JQ (1999) Hinoki (Chamaecyparis obtusa) bark, a substrate with anti-pathogen properties that suppress some root diseases of tomato. Sci Hortic 81:1–13
Yu JQ, Zhou YH, Ye SF, Huang LF (2002) 24-epibrassinolide and abscisic acid protect cucumber seedlings from chilling injury. J Hortic Sci Biotechnol 77:430–473
Yu JQ, Huang LF, Hu WH, Zhou YH, Mao WH, Ye SF, Nogues S (2004) A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J Exp Bot 55:1135–1143
Acknowledgments
This work was supported by the National Basic Research Program of China (2009CB119000), the National Natural Science Foundation of China (30771471), and National Key Project of Scientific and Technical Supporting Programs Funded by Ministry of Science & Technology of China (2008BADA6B02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogweno, J.O., Hu, W.H., Song, X.S. et al. Photoinhibition-induced reduction in photosynthesis is alleviated by abscisic acid, cytokinin and brassinosteroid in detached tomato leaves. Plant Growth Regul 60, 175–182 (2010). https://doi.org/10.1007/s10725-009-9439-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-009-9439-z