Abstract
Peltigera rufescens (Weis) Humb. with a prokaryotic photobiont Nostoc sp. and Cladina arbuscula subsp. mitis (Sandst.) Ruoss with a eukaryotic photobiont Trebouxia sp. were studied to determine the copper sensitivity of lichens with different algal symbionts. Samples growing on historic copper mine-spoil heaps at Ľubietová–Podlipa, Slovakia were assessed for physiological parameters, including total and intracellular uptake of copper, assimilation pigmentation, activity of photosystem II, ergosterol levels, thiobarbituric acid reactive substances and water-soluble protein content. Our results indicate that P. rufescens was more sensitive to copper exposure than C. arbuscula subsp. mitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Weathering of minerals in acid conditions results in the mobilization of heavy metals and other toxic elements leading to contamination of adjacent countryside. Mine waste provides a peculiar habitat for lichens, as well as plants, and particular species are frequently associated with these metal-rich sites (Purvis and Halls 1996; Bačkor and Fahselt 2004a).
It is generally assumed that the most sensitive partner to environmental pollution in the lichen symbiosis is the photobiont (Ahmadjian 1993). Indicators of metal stress on the photobiont and heavy metal tolerance previously investigated included growth rates, pigment content, mineral uptake, membrane integrity, dehydrogenase activity, photosystem II activity, levels of free proline, thiobarbituric acid reactive substances (TBARS), stress proteins and phytochelatins (Bačkor and Váczi 2002; Bačkor et al. 2003, 2004, 2006a, 2007; Pawlik-Skowrońska et al. 2002). However, knowledge of sensitivity or tolerance to heavy metals of photobionts in the lichenized state is still minimal. Photobiont involvement in lichen tolerance to heavy metals was suggested by Beck (1999) who found that all nine lichen species of the community Acarosporetum sinopicae on iron-rich rocks at “Schwarze Wand” (Austria) contained the same photobiont, Trebouxia jamesii (Hildreth & Ahmadjian) Gartner (= T. simplex Tschermak-Woess). However, further taxonomic study of metal tolerant photobionts is required, as their diversity in lichens in heavy metal rich environments appears to be unexpectedly high (Bačkor; unpublished results).
Parts of Central Slovakia (e.g., Špania dolina and Ľubietová–Podlipa) have been subjected to significant mining activity since the thirteenth century, but the earliest archeologically confirmed mining of pure copper in Europe dates from the Bronze Age. Most mine spoil heaps are between 100 and 300 years old, and mainly at the high end of this range (Bačkor and Fahselt 2004a). The lichen flora has been developing for hundreds of years and is now rich in species with both eukaryotic and prokaryotic photobionts. Human activity at these areas is currently low, the historic spoil heaps providing the most important source of heavy metal pollution with pronounced copper concentration gradients from the mine heaps to soils of adjacent meadows.
Although lichens of the genus Peltigera (including P. rufescens) that contain prokaryotic photobionts from genus Nostoc frequently grow in historical copper mining locations in Slovakia (localities Špania dolina, Rychtárová, Ľubietová, Gelnica), their presence is limited to the peripheral parts of mine-spoil heaps (soil of adjacent meadows), usually on soil with a well developed humus layer or in association with mosses. On the other hand, Cladina arbuscula subsp. mitis, although it grows along with Peltigera rufescens on the peripheral parts of mine-spoil heaps, also thrives directly on mine-spoil heaps without a well developed humus layer.
The main aim of this work was to determine physiological responses to copper excess in lichens Peltigera rufescens and Cladina arbuscula subsp. mitis which differ in type of photobiont (prokaryotic and eukaryotic). Although photobiont is probably a key element of lichen sensitivity/tolerance to heavy metals as symbiotic unit, it must be taken for consideration that photobionts are differentially sensitive to heavy metals even at level of the same species (Bačkor and Váczi 2002).
Materials and methods
Lichen material
On 5 September 2006, thalli of Peltigera rufescens (Weis) Humb. and Cladina arbuscula subsp. mitis (Sandst.) Ruoss were collected from copper mine-spoil heaps more than 200 years old in the village of Ľubietová–Podlipa (48°44′N, 19°28′E), Central Slovakia, Europe, 650 m a.s.l.. The area around Ľubietová in the NE Slovenské Stredohorie Mountains was part of the most important Cu–Fe ore fields of Slovakia. The spoil heaps are now sparsely vegetated, in part due to the high copper content, but also to soil structure, steep topography, instability, low water retention and strong sun exposure. Interestingly, only a few species of vascular plants, mainly metal tolerant species such as Agrostis vulgaris, Silene vulgaris and Rumex acetosella, are able to grow.
Substrate attributable to historical mining was considered the main source of metal pollution as study site is part of a border of Protected Landscape Area—Poľana, without recent industrial activity. Macroscopic foreign material adhering to lichen surfaces (e.g., soil particles) was removed with forceps and physiological analyses were conducted within 1 week of collection. Elemental analysis was performed within 2 weeks.
Lichen element analysis
Flame atomic absorption spectrometry was used to determine background metals (Cu, Zn, Pb, Ni, Co, Al, Fe, Sb) in lichen thalli. Lichens were dried at 70°C for 24 h and c. 100 mg of dry material was digested for 48 h in 3 ml of concentrated HNO3 (Suprapur, Merck, Darmstadt, Germany) and H2O2 (2:1, v/v) with the volume brought to 10 ml with deionized water, n = 3 (Bačkor et al. 2007). Analysis of the trace elements was performed using a Perkin–Elmer 3030B spectrometer (Perkin–Elmer Corp., Norwalk, CT, USA). Each sample was analyzed at least three times and mean values were used as one observation.
Copper stress conditions
For determination of the effects of copper concentration on selected parameters of Cladina arbuscula subsp. mitis and Peltigera rufescens, thalli were immersed in 5 mM HEPES buffer (pH 6.48). Bioavailability of copper in 5 mM HEPES buffer has been found to be high (Pawlik-Skowrońska et al. 2002). Samples (15 mg dw for assimilation pigments and lichen products, or 100 mg dw for other analyses) were exhaustively rinsed and submerged in solutions containing the following Cu concentrations: 0, 10, 25, 50, 100, 250 and 500 μM, each in total volume 50 ml, and placed for 24 h in a climatic chamber at 22°C under a 16 h photoperiod using 30 μmol m−2 s−1 PPFD from cool white fluorescent lights. Copper was supplied in the divalent form (Cu2+) as CuSO4. The dry mass of lichens was determined by weighing of sub-samples dried in an oven overnight at 90°C.
Intracellular and total copper accumulation
Thalli treated for 24 h with Cu in 5 mM HEPES buffer (pH 6.48) were removed and subsequently rinsed with 10 ml of deionized water (total copper content). Another set of identically treated thalli were washed for 20 min in 10 ml of 20 mM Na2-EDTA (analytical grade) to remove unspecifically-bound Cu, and then rinsed with 10 ml of deionized water (intracellular copper content). Samples in both sets were then mineralized by means of a mixture of 65% HNO3 and 30% H2O2 (2:1, v/v).
Metal concentrations in cells were then determined by flame atomic absorption spectroscopy (FAAS, see above for details). Three replicates for each treatment in each set were analyzed.
Pigment analysis and measurement of chlorophyll a integrity
To assess the relationship between metals and the content of assimilation pigments or chlorophyll a integrity, 15 mg of dry lichen samples previously treated by copper concentrations for 24 h and dried overnight at 30°C were weighed in conical centrifuge tubes. Secondary substances from thalli were removed by extraction with 1 ml of cool acetone for 60 min. Extraction was repeated at least three times for each sample and extracts then combined. Acetone extracts were evaporated and the residues redissolved in 1.5 ml of fresh acetone.
After evaporation of acetone overnight in the dark, phenolic-free samples were extracted in the dark for 1 h at 65°C in 3 ml of dimethyl sulfoxide (DMSO). Extracts were allowed to cool to ambient temperature, diluted 1:1 with fresh DMSO, and the absorbance, a reflection of turbidity, was checked at 750 nm with a UVI Light XTD 2 spectrophotometer (Secomam, France) to be certain that it was always less than 0.01. To assess the amount of chlorophyll, the absorbance of extracts was read at 665.1, 649.1, 435 and 415 nm on the spectrophotometer (Wellburn 1994). Absorbance was also read at 480 nm to assess total carotenoids. Chlorophyll a, chlorophyll b, chlorophyll a + b and total carotenoids were calculated using equations derived from specific absorption coefficients for pure chlorophyll a and chlorophyll b in DMSO (Wellburn 1994). Cyanolichen Peltigera rufescens do not contain chlorophyll b.
The ratios of optical densities at 435 and 415 nm (OD 435/OD 415), termed the phaeophytinization quotient, were interpreted as reflecting the ratio of chlorophyll a to phaeophytin a and provided an indication of integrity of photobiont chlorophyll (Ronen and Galun 1984). Three replicates were used.
Activity of photosystem II
Copper treated lichen samples stored for 24 h under conditions described above were dark adapted for 30 min (Bačkor et al. 2003). The potential quantum yield of photosystem II (PSII) was measured using a Plant Stress Meter (PSM Mark II, Biomonitor, SCI AB) with sensor diam. 5 mm, and results were expressed as Fv/Fm, determined as the maximal fluorescence (Fm) less the minimal fluorescence (Fo), divided by Fm of dark-adapted plants, i.e., (Fm−Fo)/Fm = Fv/Fm. Chlorophyll fluorescence parameters were determined in three different parts from each measured sample and the mean value was used as one observation. Three replicates were used.
Ergosterol determination
Lichens treated for 24 h with the above Cu concentrations in 5 mM HEPES buffer were rinsed with double distilled water. Each lichen was placed in a chilled mortar and liquid nitrogen was added. As ergosterol is sensitive to light, all steps were conducted almost in the dark. Samples were homogenized for 10 min in 99% ethanol. Extracts were transferred to 1.5 ml screw cap Eppendorf tubes and shaken in the dark at 25°C for 30 min., after which samples were vortexed and centrifuged at 10,000g for 20 min. The resulting supernatant was immediately analyzed by HPLC in a Tessek SGX C18 column 5 μm (4 × 250 mm), with flow rate 1.5 ml min−1 and methanol as the mobile phase (Dahlman et al. 2002). Total analysis time was 15 min. Detection was performed at a wavelength of 280 nm (detector Ecom LCD 2084). Ergosterol (Sigma–Aldrich, USA) was used as standard. A standard curve was constructed ranging from 1 to 200 μg ergosterol dissolved in 1 ml of ethanol. At least in three replicates were undertaken.
Thiobarbituric acid reactive substances determination
Membrane lipid peroxidation state in lichens was estimated using thiobarbituric acid reactive substances assay (TBARS) as described earlier (Kováčik and Bačkor 2007).
Thalli pre-treated with copper for 24 h were homogenized in a mortar using ice-cold 10% (w/v) trichloroacetic acid (TCA) with addition of Whatman CF/C filters (glass fiber filters which facilitate disruption of cell walls). The homogenate (2 ml final volume) was centrifuged at 6,000g for 10 min. The supernatant (1 ml) was added to 1 ml of 0.6% thiobarbituric acid (TBA) in 10% TCA. After treatment of samples in a boiling water bath for 20 min and immediate cooling in an ice bath, the mixture was again centrifuged at 6,000g for 10 min. Absorbance of the supernatant was measured at 532 nm (extinction coefficient for MDA-TBA complex 155 mM−1 cm−1) and corrected for non-specific absorption at 600 nm. Three replicates were used for each sampling site.
Protein content
Lichens treated for 24 h with copper in 5 mM HEPES buffer were homogenized in an ice-cold mortar in phosphate buffer (50 mM). After centrifugation at 15,000g at 4°C for 20 min., water-soluble protein content from supernatants were measured according to Bradford (1976) with bovine serum albumin as a calibration standard.
Statistical analysis
One-way analysis of variance and Tukey’s pairwise comparisons (MINITAB Release 11, 1996) were applied to determine the significance (P < 0.05) of differences in all measured parameters. In addition, two-way ANOVA with interactions (SPSS version 16, 2008) was applied to evaluate Cu effect (Cu), species effect (species) and interactions between Cu effect and species effect (Cu × species) where applicable.
Results
Baseline element content and copper accumulation
Baseline concentrations of selected metals in thalli of Peltigera rufescens and Cladina arbuscula subsp. mitis untreated with copper are shown in Fig. 1. Their ranked abundances in P. rufescens were: Cu ≥ Fe > Sb > Al > Zn > Ni ≥ Pb > Co, and in C. arbuscula subsp. mitis: Al ≥ Fe ≥ Sb > > Cu > Zn > Ni > Pb > Co. Although no statistical differences between the two lichen species in the accumulation of Sb, Al and Pb were found, total concentrations of Cu, Fe, Zn, Ni and Co were significantly higher in thalli of P. rufescens. ANOVA F and P values for baseline metal contents in untreated thalli of P. rufescens and C. arbuscula subsp. mitis are as follows: Cu (F = 45.1, P = 0.003), Zn (F = 200, P < 0.001), Pb (F = 5.75, P = 0.07), Ni (F = 29.6, P = 0.006), Co (F = 25.6, P = 0.007), Al (F = 0.00, P = 0.997), Fe (F = 41.2, P = 0.003), Sb (F = 3.82, P = 0.122).
Total content of selected elements in Peltigera rufescens (slash-lined bars) and Cladina arbuscula subsp. mitis (open bars) growing on historic copper-mine-spoil heaps. Values in adjacent vertical columns followed by the different letters differ significantly at P < 0.05 by Tukey’s pairwise comparisons, NS not significant, n = 3
Accumulation of copper due to laboratory treatment of thalli increased with increased concentration in HEPES buffer and differed between the two lichen species. Total copper concentration in P. rufescens reached as high as 5,000 μg/g dw when external copper concentration was 500 μM (Fig. 2a; ANOVA F = 222, P < 0.001), while in C. arbuscula subsp. mitis at the same external copper concentration it was no more than 3,000 μg/g dw (Fig. 2b; ANOVA F = 86.7, P < 0.001). Intracellularly, copper concentrations were lower and reached concentrations of 700 μg/g dw in P. rufescens (Fig. 2a; ANOVA F = 23.18, P < 0.001) and 500 μg/g dw in C. arbuscula subsp. mitis (Fig. 2b; ANOVA F = 99.2, P < 0.001) at highest external copper dose tested (500 μM).
Intracellular (open bars) and total (vertical-lined bars) copper content in lichen Peltigera rufescens (a) and lichen Cladina arbuscula subsp. mitis (b) treated for 24 h by selected copper concentrations. Values in each vertical column followed by the same letter do not differ significantly at P < 0.05 by Tukey’s pairwise comparisons, n = 3
Biological effect of copper in lichen thalli
Chlorophyll a content in Peltigera rufescens decreased after 24 h treatment with supplemental external copper (Fig. 3a), being statistically significant using 100 μM Cu. The chlorophyll a content of Cladina arbuscula subsp. mitis was also sensitive to copper in HEPES buffer and significantly decreased due to 250 μM Cu concentration (Fig. 3a). Two-way ANOVA for chlorophyll a content: Cu (F = 20.8, P < 0.001), species (F = 0.77, P = 0.388), Cu × species (F = 1.91, P = 0.11).
Chlorophyll a content (a), chlorophyll a integrity (b) and total carotenoid content (c) in lichen Peltigera rufescens (slash-lined bars) and Cladina arbuscula subsp. mitis (open bars) treated for 24 h by selected copper concentrations. Values in each vertical column followed by the same letter do not differ significantly at P < 0.05 by Tukey’s pairwise comparisons, n = 3
Chlorophyll a integrity was significantly altered by 50 μM Cu dose in P. rufescens (Fig. 3b), and by 100 μM Cu in the case of C. arbuscula subsp. mitis (Fig. 3b). Two-way ANOVA for chlorophyll a integrity: Cu (F = 61.4, P < 0.001), species (F = 2.82, P = 0.10), Cu × species (F = 5.29, P < 0.001).
Total carotenoid content was sensitive to the presence of external copper and significantly decreased as a result of 10 μM Cu in P. rufescens thalli (Fig. 3c), and 100 μM Cu in C. arbuscula subsp. mitis (Fig. 3c). Two-way ANOVA for total carotenoid content: Cu (F = 3.52, P < 0.001), species (F = 0.36, P = 0.55), Cu × species (F = 0.77, P = 0.59).
A significant decrease in the chlorophyll a/b ratio due to 25 μM external copper (Fig. 4; ANOVA F = 38.8, P < 0.001) was observed in C. arbuscula subsp. mitis, and chlorophyll b concentrations significantly increased in its photobiont cells after treatment with 50 μM Cu (Fig. 4; ANOVA F = 13.4, P < 0.001). However, chlorophyll a + b was stable during 24 h exposure to copper at all concentrations tested (Fig. 4; ANOVA F = 2.63, P = 0.064).
Chlorophyll analyses: chlorophyll a/b ratio (slash-lined bars), chlorophyll b (vertical-lined bars, mg/g dw) and chlorophyll a + b (open bars, mg/g dw) in lichen Cladina arbuscula subsp. mitis treated for 24 h by selected copper concentrations. Values in each vertical column followed by the same letter do not differ significantly at P < 0.05 by Tukey’s pairwise comparisons, NS not significant, n = 3
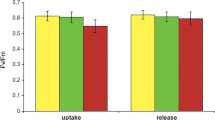
Fv/Fm decreased significantly in P. rufescens following 250 μM Cu treatment (Fig. 5a), but the photobiont of C. arbuscula subsp. mitis was tolerant to copper at any tested concentration. Although we observed decrease in Fv/Fm after the highest Cu treatments it was not strong enough to be statistically significant (Fig. 5a). Two-way ANOVA for Fv/Fm: Cu (F = 13.2, P < 0.001), species (F = 23.9, P < 0.001), Cu × species (F = 1.22, P = 0.32).
Fv/Fm (a) and ergosterol content (b) in lichen Peltigera rufescens (slash-lined bars) and Cladina arbuscula subsp. mitis (open bars) treated for 24 h by selected copper concentrations. Values in each vertical column followed by the same letter do not differ significantly at P < 0.05 by Tukey’s pairwise comparisons, n = 3
The mean ergosterol content in control samples of P. rufescens was c. 0.8 mg/g dw (Fig. 5b), and 0.6 mg/g dw in C. arbuscula subsp. mitis (Fig. 5b). The ergosterol content of both lichens significantly decreased with 50 μM Cu. Two-way ANOVA for ergosterol: Cu (F = 34.7, P < 0.001), species (F = 0.81, P = 0.37), Cu × species (F = 4.57, P = 0.01).
Concentration of TBARS in control samples of both lichen species was not statistically different, with a mean for 2 species of c. 60 μmol/g dw (Fig. 6a). In P. rufescens, TBARS increased significantly after exposure to 50 μM external copper, and in C. arbuscula subsp. mitis after 250 μM external copper. Two-way ANOVA for TBARS: Cu (F = 26.7, P < 0.001), species (F = 17.6, P < 0.001), Cu × species (F = 2.89, P = 0.03).
TBARS content (a) and content of soluble proteins (b) in lichen Peltigera rufescens (slash-lined bars) and Cladina arbuscula subsp. mitis (open bars) treated for 24 h by selected copper concentrations. Values in each vertical column followed by the same letter do not differ significantly at P < 0.05 by Tukey’s pairwise comparisons, n = 3
The concentration of soluble proteins in control thalli of P. rufescens was c. 4 mg/g dw (Fig. 6b), but in C. arbuscula subsp. mitis it was significantly lower at c. 1.6 mg/g dw. Differences in soluble protein content between the two species were significant, and externally applied copper significantly decreased protein in both at copper concentrations of 250 μM and higher. Two-way ANOVA for soluble proteins: Cu (F = 13.8, P < 0.001), species (F = 864, P < 0.001), Cu × species (F = 3.77, P = 0.01).
Discussion
Due to the low technology of mining operations in medieval times, the copper content of the spoil heaps is still very high (Bačkor and Fahselt 2004a; Banásová et al. 2006). The mean copper content of mine heaps in Central Slovakia is more than 3,600 mg/kg, and in the soils of adjacent meadows c. 150 mg/kg (Banásová et al. 2006).
It has been demonstrated previously that some lichens can accumulate metals in considerable amounts, reaching more than 5% dry weight (Seaward 1973; Purvis 1984; Bačkor and Fahselt 2004a). Foliose lichen P. rufescens does not grow directly on mine heaps and is restricted to adjacent meadows where soils are lower in copper. However it is still capable of accumulating high amounts of copper from its substrate. Higher accumulation in control thalli of P. rufescens than in control thalli of C. arbuscula subsp. mitis has also been shown for other metals, e.g., zinc, nickel, cobalt and iron, and can be probably explained by the large thallus surface area in direct contact with soil, and rhizines which are responsible for the attachment of lichens to their substrate can also be rich in metal (Goyal and Seaward 1982). In contrast, podetia of C. arbuscula subsp. mitis are attached to the substrate by only a limited surface area; hence, its significantly lower copper accumulation on Slovakian mine heaps with higher concentrations.
Chemical analyses revealed that copper was accumulated principally in extracellular sites of both lichen species. Cations, including heavy metals, can bind to extracellular sites of the mycobiont and photobiont cell walls. Cell wall exclusion of heavy metals was also confirmed by recent electron microscopic observations. By using of TEM coupled with X-ray microanalysis was demonstrated that mycobiont hyphae are for some metals (e.g., chromium and cadmium) main sites of their accumulation (Sanità di Toppi et al. 2004, 2005).
Chlorophyll a content in intact lichens (Bačkor and Zetíková 2003), as in axenic cultures of lichen photobionts (Bačkor and Dzubaj 2004), was sensitive to copper in short-term (24 h) experiments. Our results demonstrated that in the case of the eukaryotic photobiont (Trebouxia sp.) in C. arbuscula subsp. mitis, supplemental copper causes a significant concentration-dependent increase in chlorophyll b and a decrease in chlorophyll a, consistent with accelerated conversion of one to the other. Thus, chlorophyll a + b is stable parameter (Bačkor and Zetíková 2003). Because chlorophyll b is formed from chlorophyll a by the oxidation of the methyl group on ring II to the aldehyde (Chettri et al. 1998), the chlorophyll a/b ratio is more sensitive to increased copper than chlorophyll a + b (Bačkor and Váczi 2002; Bačkor and Zetíková 2003).
Chlorophyll a degradation is a frequently used parameter in lichenological studies focused on air pollution and heavy metals (Chettri et al. 1998; Bačkor and Zetíková 2003; Bačkor and Dzubaj 2004). The absorbance ratios of pigment samples (A 435 nm/A 415 nm) of c. 1.4 in control thalli indicate that chlorophyll a was not degraded (Ronen and Galun 1984). On the other hand, a significant decrease of this ratio to less than 1.0 with copper excess indicates phaeophytinisation.
Total carotenoid content was decreased under short-term exposure to increased copper concentrations. In P. rufescens thalli, the highest copper concentrations tested (250 μM and more) caused the disappearance of total carotenoids, while in thalli of C. arbuscula subsp. mitis carotenoids were still detectable at the highest copper concentrations.
The reduced Fv/Fm values measured in copper-treated lichen thalli indicate damage to PSII, consistent with long-term effect of excess copper (Bačkor and Fahselt 2004b). Branquinho et al. (1997) demonstrated that intracellular Cu > 4.0 μmol g−1 decreased chlorophyll a fluorescence in the lichen Ramalina fastigiata. Copper excess reduced Fv/Fm ratio in P. rufescens similarly as in C. arbuscula subsp. mitis, however, P. rufescens was more sensitive to the presence of additional copper at the highest copper concentration tested (500 μM).
Environmental stress on the mycobiont can be assessed from ergosterol concentration in lichens as it is the principal sterol of fungal plasma membranes. Ergosterol levels correlate with basal respiration rates of lichens (Sundberg et al. 1999) and decrease due to short term (24 h) exposure to copper excess in the aposymbiotically grown Cladonia cristatella mycobiont (Bačkor et al. 2006b).
The content of TBARS, which is positively correlated with the degree of membrane lipid peroxidation and increased ion permeability (Vavilin et al. 1998), was sensitive to copper excess. Copper is known to be a redox-reactive metal and oxidation results in formation of O2− and subsequently H2O2 and hydroxyl radical via Fenton-type reactions (Stohs and Bagchi 1995). Recently, Bačkor et al. (2007) found that TBARS production in the lichen photobiont is increased in response to copper excess. Increased production of malondialdehyde (MDA) related to damage of cell membranes in Dermatocarpon luridum subjected to excess of copper has been confirmed by Monnet et al. (2005). Content of MDA, the main constituent of TBARS, has been found to increase after 6 h of copper treatment (250 and 500 μM) in lichen D. luridum, and ranged between approximately 130 and 160 μmol/g dw. This is in agreement with results obtained in this study.
In lichens, a decline in protein content was previously demonstrated over the course of copper treatment (Monnet et al. 2006), similar to that observed in axenic photobiont cultures (Bačkor et al. 2006a). The decline of protein content due to excess copper has been confirmed in the present study. Although the concentration of soluble protein is still not a routinely used as a parameter for the assessment of environmental stress, it has been found that protein content decreased due to fumigation of lichens with SO2 (Kong et al. 1999).
The physiological experiments described here suggest that the cyanolichen P. rufescens was more sensitive to copper exposure than C. arbuscula subsp. mitis. Increased sensitivity of P. rufescens to copper excess, when compared to C. arbuscula subsp. mitis has been found in content of total carotenoids (at 250 and 500 μM Cu), Fv/Fm ratios (at 500 μM Cu), ergosterol (from 100 μM Cu) and TBARS (from 100 μM Cu). However, morphological characters of P. rufescens may allow it to accumulate significantly higher amount of copper from substrate, which make copper influence more pronounced at identical dose when compared to C. arbuscula subsp. mitis.
References
Ahmadjian V (1993) The lichen symbiosis. Wiley, New York 266 p
Bačkor M, Dzubaj A (2004) Short-term and chronic effects of copper, zinc and mercury on the chlorofyll content of four lichen photobionts and related alga. J Hattori Bot Lab 95:271–283
Bačkor M, Fahselt D (2004a) Using EDX-micronalysis and X-ray mapping to demonstrate metal uptake by lichens. Biologia 59:39–45
Bačkor M, Fahselt D (2004b) Physiological attributes of the lichen Cladonia pleurota in metal-rich and control sites near Sudbury (Ontario, Canada). Environ Exp Bot 52:149–159. doi:10.1016/j.envexpbot.2004.01.014
Bačkor M, Váczi P (2002) Copper tolerance in the lichen photobiont Trebouxia erici (Chlorophyta). Environ Exp Bot 47:11–20. doi:10.1016/S0098-8472(02)00004-7
Bačkor M, Zetíková J (2003) Effects of copper, cobalt and mercury on the chlorophyll content of lichens Cetraria islandica and Flavocetraria cucullata. J Hattori Bot Lab 93:175–187
Bačkor M, Fahselt D, Davidson RD, Wu CT (2003) Effects of copper on wild and tolerant strains of the lichen photobiont Trebouxia erici (Chlorophyta) and possible tolerance mechanisms. Arch Environ Contam Toxicol 45:159–167. doi:10.1007/s00244-002-0134-6
Bačkor M, Fahselt D, Wu CT (2004) Free proline content is positively correlated with copper tolerance of the lichen photobiont Trebouxia erici (Chlorophyta). Plant Sci 167:151–157. doi:10.1016/j.plantsci.2004.03.012
Bačkor M, Gibalová A, Buďová J, Mikeš J, Solár P (2006a) Cadmium-induced stimulation of stress-protein hsp70 in lichen photobiont Trebouxia erici. Plant Growth Regul 50:159–164. doi:10.1007/s10725-006-9112-8
Bačkor M, Pawlik-Skowrońska B, Tomko J, Buďová J, Sanità di Toppi L (2006b) Response to copper stress in aposymbiotically grown lichen mycobiont mykobiont Cladonia cristatella: uptake, viability, ergosterol and production of non-protein thiols. Mycol Res 110:994–999. doi:10.1016/j.mycres.2006.05.007
Bačkor M, Váczi P, Barták M, Buďová J, Dzubaj A (2007) Uptake, photosynthetic characteristics and membrane lipid peroxidation levels in the lichen photobiont Trebouxia erici exposed to copper and cadmium. Bryologist 110:100–107. doi:10.1639/0007-2745(2007)110[100:UPCAML]2.0.CO;2
Banásová V, Horák O, Čiamporová M, Nadubinská M, Lichtscheidl I (2006) The vegetation of metalliferous and non-metalliferous grasslands in two former mine regions in Central Slovakia. Biologia 61:433–439. doi:10.2478/s11756-006-0073-1
Beck A (1999) Photobiont inventory of a lichen community growing on heavy-metal-rich rock. Lichenologist 31:501–510
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Branquinho C, Brown DH, Catarino F (1997) The cellular location of Cu in lichens and its effects on membrane integrity and chlorophyll fluorescence. Environ Exp Bot 38:165–179. doi:10.1016/S0098-8472(97)00015-4
Chettri MK, Cook CM, Vardaka E, Sawidis T, Lanaras T (1998) The effect of Cu, Zn and Pb on the chlorophyll content of the lichen Cladonia convoluta and Cladonia rangiformis. Environ Exp Bot 39:1–10. doi:10.1016/S0098-8472(97)00024-5
Dahlman L, Zetherström M, Sundberg B, Näsholm T, Palmqvist K (2002) Measuring ergosterol and chitin in lichens. In: Kranner I, Beckett R, Varma A (eds) Protocols in lichenology: culturing, biochemistry, ecophysiology and use in biomonitoring. Springer-Verlag, Heidelberg, pp 348–362
Goyal R, Seaward MRD (1982) Metal uptake in terricolous lichens. III. Translocation in the thallus of Peltigera canina. New Phytol 90:85–98. doi:10.1111/j.1469-8137.1982.tb03244.x
Kong FX, Hu W, Chao SY, Sang WL, Wang LS (1999) Physiological responses of the lichen Xanthoparmelia mexicana to oxidative stress of SO2. Environ Exp Bot 42:201–209. doi:10.1016/S0098-8472(99)00034-9
Kováčik J, Bačkor M (2007) Changes of phenolic metabolism and oxidative status in nitrogen-deficient Matricaria chamomilla plants. Plant Soil 297:255–265. doi:10.1007/s11104-007-9346-x
Monnet F, Bordas F, Deluchat V, Chatenet P, Botineau M, Baudu M (2005) Use of the aquatic lichen Dermatocarpon luridum as bioindicator of copper pollution: accumulation and cellular distribution tests. Environ Pollut 138:455–461. doi:10.1016/j.envpol.2005.04.019
Monnet F, Bordas F, Deluchat V, Baudu M (2006) Toxicity of copper excess on the lichen Dermatocarpon luridum: antioxidant enzyme activities. Chemosphere 65:1806–1813. doi:10.1016/j.chemosphere.2006.04.022
Pawlik-Skowrońska B, Sanità di Toppi L, Favali MA, Fossati F, Pirszel J, Skowroński T (2002) Lichens respond to heavy metals by phytochelatin synthesis. New Phytol 156:95–102. doi:10.1046/j.1469-8137.2002.00498.x
Purvis OW (1984) The occurrence of copper oxalate in lichens growing on copper sulphide-bearing rocks in Scandinavia. Lichenologist 16:197–204. doi:10.1017/S0024282984000347
Purvis OW, Halls C (1996) A review of lichens in metal-enriched environments. Lichenologist 28:571–601
Ronen R, Galun M (1984) Pigment extraction from lichens with dimethyl sulfoxide (DMSO) and estimation of chlorophyll degradation. Environ Exp Bot 24:239–245. doi:10.1016/0098-8472(84)90004-2
Sanità di Toppi L, Musetti R, Marabottini R, Corradi MG, Vattuone Z, Favali MA, Badiani M (2004) Responses of Xanthoria parietina thalli to environmentally relevant concentrations of hexavalent chromium. Funct Plant Biol 31:329–338. doi:10.1071/FP03171
Sanità di Toppi L, Marabottini R, Vattuone Z, Musetti R, Favali MA, Sorgonà A, Badiani M (2005) Cell wall immobilization and antioxidant status of Xanthoria parietina thalli exposed to cadmium. Funct Plant Biol 32:611–618. doi:10.1071/FP04237
Seaward MRD (1973) Lichen ecology of the Scunthorpe Heathlands I. Mineral accumulation. Lichenologist 5:423–433. doi:10.1017/S0024282973000472
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18:321–336. doi:10.1016/0891-5849(94)00159-H
Sundberg B, Ekblad A, Nasholm T, Palmqvist K (1999) Lichen respiration in relation to active time, temperature, nitrogen and ergosterol concentrations. Funct Ecol 13:119–125. doi:10.1046/j.1365-2435.1999.00295.x
Vavilin DV, Ducruet JM, Matorin DN, Venediktov PS, Rubin AB (1998) Membrane lipid peroxidation, cell viability and photosystem II activity in the green alga Chlorella pyrenoidosa subjected to various stress conditions. J Photochem Photobiol B 42:233–239. doi:10.1016/S1011-1344(98)00076-1
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolutions. J Plant Physiol 144:307–313
Acknowledgments
This work was financially supported by Slovak Research and Development Agency (APVV) under the contracts APVT-20-003004 and Slovak Grant Agency (VEGA 1/1285/04) to MB. Thanks are expressed to Prof. Dianne Fahselt (UWO, Canada) and Prof. M. R. D. Seaward (Bradford University, UK) for comments on the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Bačkor, M., Kováčik, J., Dzubaj, A. et al. Physiological comparison of copper toxicity in the lichens Peltigera rufescens (Weis) Humb. and Cladina arbuscula subsp. mitis (Sandst.) Ruoss. Plant Growth Regul 58, 279–286 (2009). https://doi.org/10.1007/s10725-009-9376-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-009-9376-x