Abstract
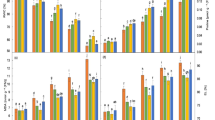
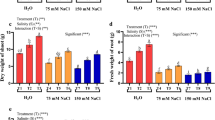
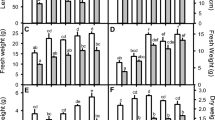
This study investigates the role of salicylic acid (SA), hydrogen peroxide (H2O2) and calcium chloride (CaCl2) singly or in combination, in inducing naked oat plant tolerance to sodium chloride (NaCl). Two-week-old naked oat plants were pretreated with both single and double of 0.5 mM SA, 0.5 mM H2O2 and 5 mM CaCl2 by adding them to the culture solution for 24 h. At the end of the pretreatment, the plants were subjected to 200 mM NaCl exposure for 7 days. Data were collected on plant biomass, H2O2 level, antioxidant enzyme activity, non-enzymatic antioxidant content and malondialdehyde (MDA) content. Results showed that exposure to salt significantly inhibited plant growth, and the shoot and root dry weights were reduced 47.5% and 63.4%, and the H2O2 levels elevated 5.8 and 2.4 times in comparison with those in the control, respectively. Under the saline stress, the activities of superoxide dismutase (SOD) and catalase (CAT) were induced, but the contents of ascorbic acid (AA) and glutathione (GSH) decreased, and MDA largely accumulated. The various pretreatments efficiently counteracted the salt-caused growth inhibition, especially with H2O2 + CaCl2 the shoot and root dry weights reduced only 9.4% and 24.4% of the non salt-stressed plants. The determination of endogenous H2O2 level demonstrated that the pretreatments induced H2O2 accumulation, with H2O2 + CaCl2 being most efficient, but the effect was transient. After 7 days of saline stress, the H2O2 contents in the pretreated shoots and roots accounted for 23.7–41.8% and 31.7–57.3% of the non-pretreated plants, varying according to the different pretreatments. Under saline stress, SOD and CAT further increased, AA and GSH maintained higher levels and MDA decreased in the pretreated plants compared to the untreated plants. With application of diphenylene iodonium (DPI) during the pretreatment, which inhibited the accumulation of H2O2, the ameliorative effect of the pretreatment on salt-caused plant growth inhibition was reduced. However, applied DPI at the immediate end of the pretreatment did not alter its favorable role, indicating a H2O2 peak formed at the early time of saline stress might play an important role in regulating plant tolerance to saline stress.









Similar content being viewed by others
References
Aebi HE (1983) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analyses. Weinheim, Verlag Chemie, pp 273–282
Agarwal S, Sairam RK, Srivastava GC, Tyagi A, Meena RC (2005) Role of ABA, salicylic acid, calcium and hydrogen peroxide on antioxidant enzymes induction in wheat seedlings. Plant Sci 169:559–570
Alvarez M, Pennell R, Meijer P, Ishikawa A, Dixon R, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92:773–784
Arfan M, Athar HR, Ashraf M (2007) Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring naked oat cultivars under salt stress? J Plant Physiol 64:685–694
Azevedo Neto AD, Prisco JT, Eneas-Filho J, Medeiros JV, Gomes-Filho E (2005) Hydrogen peroxide pre-treatment induces salt-stress acclimation in maize plants. J Plant Physiol 162:1114–1122
Beauchamp C, Fridovich J (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beckman KB, Ames BN (1997) Oxidative decay of DNA. J Biol Chem 272:19633–19636
Berlett BS, Stadtman ER (1997) Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272:20313–20316
Borsani O, Valpuesta V, Botella MA (2001) Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol 126:1024–1030
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen WP, Li PH (2001) Chilling induced Ca2+ over load enhances production of active oxygen species in maize (Zea mays L.) cultured cells: the effect of abscisic acid treatment. Plant Cell Environ 24:791–800
Dat J, Vandenabeele S, Vranová E, van Montagu M, Inzé D, van Breusegem F (2000) Dual action of active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
Demidchik V, Tester M (2002) Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiol 128:379–387
Desikan R, Neill SJ, Hancock JT (2000) Hydrogen peroxide-induced gene expression in Arabidopsis thaliana. Free Radic Biol Med 28:773–778
Elphick CH, Sanders D, Maathuis FJM (2001) Critical role of divalent cations and Na+ efflux in Arabidopsis thaliana salt tolerance. Plant Cell Environ 24:733–740
Ganesan V, Thomas G (2001) Salicylic acid response in rice: influence of salicylic acid on H2O2 accumulation and oxidative stress. Plant Sci 160:1095–1106
Gapper C, Dolan L (2006) Control of plant development by reactive oxygen species. Plant Physiol 141:341–345
Grant M, Brown I, Adams S, Knight M, Ainslie A, Mansfield J (2000) The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J 23:441–450
Griffith OW, Meister A (1979) Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (s-n-butyl-homocysteine sulfoximine). J Biol Chem 254:7558–7560
Gunes A, Inal A, Alpaslan M, Eraslan F, Bagci EG, Cicek N (2007) Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J Plant Physiol 164:728–736
Kadota Y, Goh T, Tomatsu H, Tamauchi R, Higashi K, Muto S, Kuchitsu K (2004) Cryptogein-induced initial events in tobacco BY-2 cells: pharmacological characterization of molecular relationship among cytosolic Ca2+ transients, anion efflux and production of reactive oxygen species. Plant Cell Physiol 45:160–170
Keller T, Schwager H (1977) Air pollution and ascorbic acid. Eur J Forest Pathol 7:338–350
LaHaye PA, Epstein E (1969) Salt tolerance in plants: enhancement with calcium. Science 166:395–396
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48:251–275
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Molina A, Bueno P, Marlin MC, Rodriguez-Rosales MP, Belver A, Venema K, Danaire JP (2002) Involvement of endogenous salicylic acid content, lipoxygenase and antioxidant enzyme activities in the response of tomato cell suspension cultures to NaCl. New Phytol 156:409–415
Montillet JL, Chamnongpol S, Rustérucci C, Dat J, Van de Cotte B, Agnel JP, Battesti C, Inzé D, Van Breusegem F, Triantaphylidès C (2005) Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol 138:1516–1526
Mukherjee SP, Choudhuri MA (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:166–170
Mullineaux P, Karpinski S (2002) Signal transduction in response to excess light: getting out of the chloroplast. Curr Opin Plant Biol 5:43–48
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663
Orozco-Cárdenas ML, Narváez-Vásquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13:179–191
Pan Y, Wu LJ, Yu ZL (2006) Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul 49:157–165
Pasternak D. (1987) Salt tolerance and crop production: a comprehensive approach. Annu Rev Phytopathol 25:271–291
Pitman MG, Läuchli A (2002) Global impacts of salinity and agricultural ecosystem. In: A Läuchli U Lüttge (eds) Salinity: environment-plants-molecules. Kluwer Academic, Dordrecht, The Netherlands, pp 3–20
Potocký M, Jones MA, Bezvoda R, Smirnoff N, Zárský V (2007) Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol 174:742–751
Radic S, Radic-Stoikovic M, Pevalek-Kozlina B (2006) Influence of NaCl and mannitol on peroxidase activity and lipid peroxidation in Centaurea ragusina L. roots and shoots. J Plant Physiol 163:1284–1292
Rao MV, Paliyath G, Ormrod DP, Murr DP, Watkins CB (1997) Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes. Salicylic acid-mediated oxidative damage requires H2O2. Plant Physiol 115:137–149
Rodríguez A, Grunberg K, Taleisnik E (2002) Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiol 129:1627–1632
Rodríguez A, Córdoba A, Ortega L, Taleisnik E (2004) Decreased reactive oxygen species concentration in the elongation zone contributes to the reduction in maize leaf growth under salinity. J Exp Bot 55:1383–1390
Rodríguez A, Lascano HR, Bustos D, Taleisnik E (2007) Salinity-induced decrease in NADPH oxidase activity in the maize leaf blade elongation zone. J Plant Physiol 164:223–230
Saijo Y, Hata S, Kyozuka J, Shimanoto K, Izui K (2000) Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J 23:319–327
Saijo Y, Kinoshita N, Ishiyama K, Hata S, Kyozuka J, Hayakawa T, Nakamura T, Shimamoto K, Yamaya T, Izui K (2001) A Ca2+-dependent kinase that endows rice plants with cold-and salt-stress tolerance functions in vascular bundles. Plant Cell Physiol 42:1228–1233
Shabala S, Shabala L, Van Volkenburgh E (2003) Effect of calcium on root development and root ion fluxes in salinized barley seedlings. Funct Plant Biol 25:608–616
Shabala S, Shabala L, Van Volkenburgh E, Newman I (2005) Effect of divalent cations on ion fluxes and leaf photochemistry in salinized barley leaves. J Exp Bot 56:1369–1378
Shabala S, Demidchik V, Shabala L, Cuin TA, Smith SJ, Davies JM, Newman IA (2006) Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol 141:1653–1665
Shalata A, Tal M (1998) The effect of salt stress on lipid peroxidation and antioxidants in the leaf of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol Plant 104:167–174
Stevens J, Senaratna T, Sivasithamparam K (2006) Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): associated changes in gas exchange, water relations and membrane stabilization. Plant Growth Regul 49:77–83
Sumithra K, Jutur PP, Dalton Carmel B, Reddy AR (2006) Salinity-induced changes in two cultivars of Vigna radiata: responses of antioxidative and proline metabolism. Plant Growth Regul 50:11–22
Tsai YC, Hong CY, Liu LF, Kao CH (2005) Expression of ascorbate peroxidase and glutathione reductase in roots of rice seedlings in response to NaCl and H2O2. J Plant Physiol 162:291–299
Uchida A, Jagendorf AT, Hibino T, Takabe T, Takabe T (2002) Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci 63:515–523
Valderrama R, Corpas FJ, Carreras A, Gomez-Rodriguez MV, Chaki M, Pedrajas JR, Fernandez-Ocana A, Del Rio LA, Barroso JB (2006) The dehydrogenase-mediated recycling of NADPH is a key antioxidant system against salt-induced oxidative stress in olive plants. Plant Cell Environ 29:1449–1459
Wahid A, Perveen M, Gelani S, Basra SM (2007) Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol 164:283–294
Yang T, Poovaiah BW (2002) Hydrogen peroxide homeostasis: Activation of plant catalase by calcium/calmodulin. Proc Natl Acad Sci USA 99:4097–4102
Yang Y, Xu S, An L, Chen N (2007) NADPH oxidase-dependent hydrogen peroxide production, induced by salinity stress, may be involved in the regulation of total calcium in roots of wheat. J Plant Physiol 164:1429–1435
Zhao KF, Fan H, Song J (2002) Species, types, vegetation of halophytes in China and its economic potential. In: Liu XJ, Liu MY (eds) Utilization of halophytes and sustainable development of local agriculture. China Meteorological Press, Beijing, pp 1–9
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445
Zhu XJ, Yang JS, Liang YC, Lou YS, Yang XY (2004) Effects of exogenous calcium on photosynthesis and its related physiological characteristics of rice seedlings under salt stress. Sci Agric Sin 7:1497–1503 (in Chinese)
Acknowledgements
This research was supported by the National Natural Science Foundation of China (30570445), Natural Science Foundation of Liaoning Province (No. 20021022), Tackle Key Problem of Science and Technology, Education Department of Liaoning Province (2004D005) and Director Foundation of Experimental Centre, Shenyang Normal University (SY200406).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, Q., Xu, X., Zhao, Y. et al. Salicylic acid, hydrogen peroxide and calcium-induced saline tolerance associated with endogenous hydrogen peroxide homeostasis in naked oat seedlings. Plant Growth Regul 54, 249–259 (2008). https://doi.org/10.1007/s10725-007-9247-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-007-9247-2