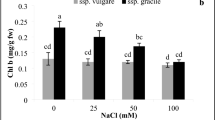
Abstract
The effects of salinity and drought on the antioxidative system (SOD, POD, CAT) were studied in liquorice seedlings (Glycyrrhiza uralensis Fisch). The results showed that both salt and drought stresses could induce oxidative stress, as indicated by the increase level of lipid peroxidation. The activities of SOD and POD were up-regulated by salt and drought stress, while CAT activity decreased. An additional MnSOD isoenzyme was detected in liquorice subjected to 2%NaCl stress. The data also showed that although the activity of SOD was differentially influenced by drought and salinity, the changes of antioxidant enzyme activities subjected to drought stress follow a pattern similar to that subjected to salt stress, indicating that similar defensive systems might be involved in the oxidative stress injury in liquorice.



Similar content being viewed by others
Abbreviations
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- POD:
-
Peroxidase
- MDA:
-
Malondialdehyde
- RWC:
-
Relative water content
- PVP:
-
Polyvinylpyrrolidon
- EDTA:
-
Ethylenediaminetetraacetic acid
- CK:
-
Control
- TEMED:
-
N,N,N′,N′-Tetramethylethylenediamine
- PEG:
-
Polyethylene glycol
- PBS:
-
Phosphate buffer saline
- NBT:
-
Nitroblue tetrazolium
- dw:
-
Distilled water
References
Bannister JV, Bannister WH, Rotilio G (1987) Aspects of the structure, function and applications of superoxide dismutase. Crit Rev Biochem 22:111–180
Baum JA, Scandalios JG (1981) Isolation and characterization of the cytosolic and mitochondrial superoxide dismutases of maize. Arch Biochem Biophys 206:249–264
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bowler C, Van Camp W, Van Montagu M, Inzé D (1994) Superoxide dismutase in plants. Crit Rev Plant Sci 13:199–218
Bradford MN (1976) A rapid and sensitive method for the quantiation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Creissen GP, Edwards AE, Mullineaux PM (1994) Glutathione reductase and ascorbate peroxidase. In: Foyer CH, Mullineaux PM (eds) Causes of photooxidative stress and amelioration of defense systems in plants. CRC Press, Boca Raton, pp 343–364
Del Río LA, Sandalio LM, Palma JM, Bueno P, Corpas FJ (1992) Metabolism of oxygen radicals in peroxisomes and cellular implications. Free radic biol med 13:557–580
Fernández VM, Sevilla F, López Gorgé J, Gómez M, Del Río LA (1982) Evidence for manganese (III) binding to the mangano superoxide dismutase from a higher plant (Pisum sativum L.). J Inorg Biochem 16:79–84
Fridovich I (1986) Superoxide dismutases. In: Meister A (ed) Advances in enzymology and related areas of molecular biology, vol 58, Wiley, New York, pp 61–97
Gómez M, Hernández JA, Jiménez A, Del Río LA, Sevilla F (1999) Differential response of antioxidative system of chloroplasts and mitochondria to long-term NaCl stress of pea plants. Free Radic Res 31:11–18
Gosset DR, Millhollon EP, Lucas MC (1994) Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci 34:706–714
Gueta Dahan Y, Yaniv Z, Zilinskas BA, Hayyim Ben G (1997) Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in citrus. Planta 203:460–469
Hernández Corpas FJ, Gómez M, Del Río LA, Sevilla F (1993) Salt-induced oxidative stress mediated by activated oxygen species in pea leaf mitochondria. Physiol Plant 89:103–110
Hernández JA, Jiménez A, Mullineaux P, Sevilla F (2000) Tolerance of pea (Pisum sativum L) to long-term salt stress is associated with the induction of antioxidant defences. Plant Cell Environ 23:853–862
Hsiao TC, (1973) Plant response to water stress. Annu Rev Plant Physiol 126:1266–1274
Khan MH, Singha KLB, Panda SK (2002) Changes in antioxidant levels in Oryza sativa L. roots subjected to NaCl salinity stress. Acta Physiol Plant 24:145–148
Kochba J, Lavee S, Roy Spiegel P (1977) Differences in peroxidase activity and isoenzymes in embryogenic and non-embryogenic “Shamouti” orange ovular callus lines. Plant Cell Physiol 18:463–467
Kröniger W, Rennenberg H, Tadros MH, Polle A (1995) Purification and properties of manganese superoxide dismutase from Norway spruce (Picea abies L. Karst). Plant Cell Physiol 36:191–196
Luna M, Badiani M, Felice M, Artemi F, Germanni G (1985) Selective enzyme inactivation under water stressin maize (Zea mays L.) and wheat (Triticum aestium L.) seedlings. Environ Exp Bot 25:153–156
Maribel L, Dionisio Sese and Satoshi Tobita (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Moran JF, Becana M, Iturbe-Ormaetxe I, Frechilla S, Klucas RVP, Aparicio T (1994) Drought induces oxidative stress in pea plants. Planta 194:346–352
Olmos E, Hernández JA, Sevilla F, Hellín E (1994) Induction of several antioxidant enzymes in the selection of a salt-tolerant cell line of Pisum sativum. J Plant Physiol 144:594–598
Palatnik Javier F, Estela Valle M, María Federico L, Leonardo D. Gómez, Mariana N. Melchiorre, Antonio Díaz Paleo, Néstor Carrillo, Alberto Acevedo (2002) Status of antioxidant metabolites and enzymes in catalase-deficient mutant of barley (Hordeum ulgare L.). Plant Sci 162:363–371
Quartacci MF, Navaro L, F (1992) Water stress a free radical mediated changes in sunflower seedlings. Plant Physiol 142:621–625
Ruth GA, Neval E, Lenwood SH (2002) Role of superoxide dismutase (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Sandalio LM, Dalurzo HC, Gómez M, Romero Puertas MC, Del Río LA (2001) Oxidative metabolism of pea plants. J Exp Bot 52:2115–2126
Scalet M, Federice R, Guido MC, Manes F (1995) Peroxidase activity and polyamine changes in response to ozone and simulated acid rain in Aleppo pine needles. Environ Exp Bot 35:417–425
Scandalios JG (1993) Oxygen stress and superoxide dismutases. Plant Physiol 101:7–12
Sevilla F, López Gorgé J, Gómez M, Del Río LA (1980) Manganese superoxide dismutase from a higher plant: purification of a new Mn-containing enzyme. Planta 150:153–157
Sevilla F, López Gorgé J, Gómez M, Del Río LA (1982) Characterization of a manganese superoxide dismutase from the higher plantPisum sativum L. Plant Physiol 70:1321–1326
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Steinman H (1982) Superoxide dismutases: Protein chemistry and structure-function relationships. In: Oberley LW (ed) Superoxide Dismutase, vol. I, CRC Press, Boca Raton, Florida, pp 11–68
Streller S, Krömer S, Wingsle G (1994) Isolation and purification of mitochondrial Mn-superoxide dismutase from the gymnosperm Pinus sylvestris L. Plant Cell Physiol 35:859–867
Van Camp W, Hérouart D, Willekens H, Takahashi H, Saito K, Van Montagu M, Inzé D (1996) Tissue-specific activity of two manganese superoxide dismutase promoters in transgenic tobacco. Plant Physiol 112:525–535
Van Rensburg L, Krüger GHJ (1994) Evaluation of components of oxidative stress metabolism for use in selection of drought tolerant cultivars of Nicotiana tabacum L. J Plant Physiol 143:730–737
Whetherley PE (1950) Studies in the water relations of cotton plants I The field measurement of water deficit in leaves. New Phyrol 49:81–87
Zhu D, Scandalios JG (1993) The maize mitochondrid manganese superoxide dismutases (MnSOD’s) are a differentially expressed multigene family. Proc Natl Acad Sci USA 90:9310–9314
Zhang FM, Li XY (1997) Relationship between liquorice morphological variation and environment. Environmental Protection of Xinjiang 19:33–37
Zhang J, Kirkham MB (1995) Water relations of water stressed, split-root C4 (Sorghum bicolor; Poaceae) and C3 (Helianthus annuus; Asteraceae) plants. Am J Bot 82:1220–1229
Zhang PY, Peng ZX (1960) Liquorice in Northwest of China. Journal of Lanzhou University 1:57–87
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Acknowledgements
This research was supported by National High-Tech Research and Development Plan of China under grant No. 2002AA327070 and No.2004AA326060.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pan, Y., Wu, L.J. & Yu, Z.L. Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul 49, 157–165 (2006). https://doi.org/10.1007/s10725-006-9101-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-006-9101-y