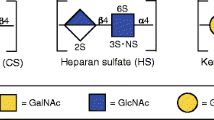
Abstract
The complex microenvironment that surrounds hematopoietic stem cells (HSCs) in the bone marrow niche involves different coordinated signaling pathways. The stem cells establish permanent interactions with distinct cell types such as mesenchymal stromal cells, osteoblasts, osteoclasts or endothelial cells and with secreted regulators such as growth factors, cytokines, chemokines and their receptors. These interactions are mediated through adhesion to extracellular matrix compounds also. All these signaling pathways are important for stem cell fates such as self-renewal, proliferation or differentiation, homing and mobilization, as well as for remodeling of the niche. Among these complex molecular cues, this review focuses on heparan sulfate (HS) structures and functions and on the role of enzymes involved in their biosynthesis and turnover. HS associated to core protein, constitute the superfamily of heparan sulfate proteoglycans (HSPGs) present on the cell surface and in the extracellular matrix of all tissues. The key regulatory effects of major medullar HSPGs are described, focusing on their roles in the interactions between hematopoietic stem cells and their endosteal niche, and on their ability to interact with Heparin Binding Proteins (HBPs). Finally, according to the relevance of HS moieties effects on this complex medullar niche, we describe recent data that identify HS mimetics or sulfated HS signatures as new glycanic tools and targets, respectively, for hematopoietic and mesenchymal stem cell based therapeutic applications.


Similar content being viewed by others
References
Couchman, J.: Transmembrane signaling proteoglycans. Annu. Rev. Cell Dev. Biol. 26, 89–114 (2010)
Kjellen, L., Lindahl, U.: Proteoglycans: structures and interactions. Annu. Rev. Biochem. 60, 443–475 (1991)
Kreuger, J., et al.: Interactions between heparan sulfate and proteins: the concept of specificity. J. Cell Biol. 174(3), 323–327 (2006)
Gallagher, J.T.: Heparan sulfate: growth control with a restricted sequence menu. J. Clin. Invest. 108(3), 357–361 (2001)
Kreuger, J., et al.: Fibroblast growth factors share binding sites in heparan sulphate. Biochem. J. 389(Pt 1), 145–150 (2005)
Lindahl, U., Kusche-Gullberg, M., Kjellen, L.: Regulated diversity of heparan sulfate. J. Biol. Chem. 273(39), 24979–24982 (1998)
Lindahl, U., Li, J.P.: Interactions between heparan sulfate and proteins-design and functional implications. Int. Rev. Cell Mol. Biol. 276, 105–159 (2009)
Kreuger, J., et al.: Sequence analysis of heparan sulfate epitopes with graded affinities for fibroblast growth factors 1 and 2. J. Biol. Chem. 276(33), 30744–30752 (2001)
Huynh, M.B., et al.: Glycosaminoglycans from aged human hippocampus have altered capacities to regulate trophic factors activities but not Abeta42 peptide toxicity. Neurobiol. Aging. 33(5), 1005 e11–1005 e22 (2012)
Huynh, M.B., et al.: Age-related changes in rat myocardium involve altered capacities of glycosaminoglycans to potentiate growth factor functions and heparan sulfate-altered sulfation. J. Biol. Chem. 287(14), 11363–11373 (2012)
Negroni, E., et al.: Glycosaminoglycan modifications in Duchenne muscular dystrophy: specific remodeling of chondroitin sulfate/dermatan sulfate. J. Neuropathol. Exp. Neurol. 73(8), 789–797 (2014)
Chevalier, F., et al.: A fine structural modification of glycosaminoglycans is correlated with the progression of muscle regeneration after ischaemia: towards a matrix-based therapy? Eur. Cell. Mater. 30, 51–68 (2015)
Sugahara, K., Kitagawa, H.: Heparin and heparan sulfate biosynthesis. IUBMB Life. 54(4), 163–175 (2002)
Berninsone, P., Hirschberg, C.B.: Nucleotide sugars, nucleotide sulfate, and ATP transporters of the endoplasmic reticulum and Golgi apparatus. Ann. N. Y. Acad. Sci. 842, 91–99 (1998)
Fuller, M., et al.: A defect in exodegradative pathways provides insight into endodegradation of heparan and dermatan sulfates. Glycobiology. 16(4), 318–325 (2006)
Esko, J.D., Selleck, S.B.: Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 71, 435–471 (2002)
Bishop, J.R., Schuksz, M., Esko, J.D.: Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 446(7139), 1030–1037 (2007)
Iozzo, R.V.: Matrix proteoglycans: from molecular design to cellular function. Annu. Rev. Biochem. 67, 609–652 (1998)
Bix, G., Iozzo, R.V.: Novel interactions of perlecan: unraveling perlecan's role in angiogenesis. Microsc. Res. Tech. 71(5), 339–348 (2008)
Hohenester, E., et al.: The crystal structure of a laminin G-like module reveals the molecular basis of alpha-dystroglycan binding to laminins, perlecan, and agrin. Mol. Cell. 4(5), 783–792 (1999)
Halfter, W., et al.: Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J. Biol. Chem. 273(39), 25404–25412 (1998)
Afratis, N.A., et al.: Syndecans - key regulators of cell signaling and biological functions. FEBS J. 284(1), 14 (2017)
Fico, A., Maina, F., Dono, R.: Fine-tuning of cell signaling by glypicans. Cell. Mol. Life Sci. 68(6), 6 (2011)
Fuster, M.M., Wang, L.: Endothelial heparan sulfate in angiogenesis. Prog. Mol. Biol. Transl. Sci. 93, 179–212 (2010)
Sarrazin, S., Lamanna, W.C., Esko, J.D.: Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 3(7) (2011). doi:10.1101/cshperspect.a004952
Lin, X.: Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 131(24), 6009–6021 (2004)
Schofield, R.: The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 4(1–2), 7–25 (1978)
Asada, N., Katayama, Y.: Regulation of hematopoiesis in endosteal microenvironments. Int. J. Hematol. 99(6), 679–684 (2014)
Kiel, M.J., Morrison, S.J.: Uncertainty in the niches that maintain haematopoietic stem cells. Nat. Rev. Immunol. 8(4), 290–301 (2008)
Stier, S., et al.: Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J. Exp. Med. 201(11), 1781–1791 (2005)
Zhang, J., et al.: Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 425(6960), 836–841 (2003)
Arai, F., et al.: Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 118(2), 149–161 (2004)
Yoshihara, H., et al.: Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 1(6), 685–697 (2007)
Mendez-Ferrer, S., et al.: Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 466(7308), 829–834 (2012)
Levesque, J.P., Helwani, F.M., Winkler, I.G.: The endosteal 'osteoblastic' niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 24(12), 1979–1992 (2010)
Bonewald, L.F.: The amazing osteocyte. J. Bone Miner. Res. 26(2), 229–238 (2011)
Tatsumi, S., et al.: Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 5(6), 464–475 (2007)
Scadden, D.T.: The stem-cell niche as an entity of action. Nature. 441(7097), 1075–1079 (2006)
Kollet, O., et al.: Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat. Med. 12(6), 657–664 (2006)
Sugiyama, T., et al.: Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 25(6), 977–988 (2006)
Sacchetti, B., et al.: Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 131(2), 324–336 (2007)
Heissig, B., et al.: Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 109(5), 625–637 (2002)
Avecilla, S.T., et al.: Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 10(1), 64–71 (2004)
Purton, L.E., Scadden, D.T.: The hematopoietic stem cell niche. Harvard Stem Cell Institute, Cambridge (2008)
Adams, G.B., Scadden, D.T.: The hematopoietic stem cell in its place. Nat. Immunol. 7(4), 333–337 (2006)
Friel, J., et al.: Diverse isoforms of colony-stimulating factor-1 have different effects on the development of stroma-dependent hematopoietic cells. J. Cell. Physiol. 204(1), 247–259 (2005)
Zandstra, P.W., Lauffenburger, D.A., Eaves, C.J.: A ligand-receptor signaling threshold model of stem cell differentiation control: a biologically conserved mechanism applicable to hematopoiesis. Blood. 96(4), 1215–1222 (2000)
Doran, M.R., et al.: Surface-bound stem cell factor and the promotion of hematopoietic cell expansion. Biomaterials. 30(25), 4047–4052 (2009)
Bhatia, M., et al.: Quantitative analysis reveals expansion of human hematopoietic repopulating cells after short-term ex vivo culture. J. Exp. Med. 186(4), 619–624 (1997)
Miller, C.L., Eaves, C.J.: Expansion in vitro of adult murine hematopoietic stem cells with transplantable lympho-myeloid reconstituting ability. Proc. Natl. Acad. Sci. U. S. A. 94(25), 13648–13653 (1997)
Conneally, E., et al.: Expansion in vitro of transplantable human cord blood stem cells demonstrated using a quantitative assay of their lympho-myeloid repopulating activity in nonobese diabetic-scid/scid mice. Proc. Natl. Acad. Sci. U. S. A. 94(18), 9836–9841 (1997)
Ueda, T., et al.: Expansion of human NOD/SCID-repopulating cells by stem cell factor, Flk2/Flt3 ligand, thrombopoietin, IL-6, and soluble IL-6 receptor. J. Clin. Invest. 105(7), 1013–1021 (2000)
Ehninger, A., Trumpp, A.: The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J. Exp. Med. 208(3), 421–428 (2011)
Thoren, L.A., et al.: Kit regulates maintenance of quiescent hematopoietic stem cells. J. Immunol. 180(4), 2045–2053 (2008)
Zhang, C.C., Sadek, H.A.: Hypoxia and metabolic properties of hematopoietic stem cells. Antioxid. Redox Signal. 20(12), 1891–1901 (2012)
Chen, J., et al.: Erythropoietin-dependent autocrine secretion of tumor necrosis factor-alpha in hematopoietic cells modulates proliferation via MAP kinase--ERK-1/2 and does not require tyrosine docking sites in the EPO receptor. Exp. Cell Res. 298(1), 155–166 (2004)
Sitnicka, E., et al.: Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity. 17(4), 463–472 (2002)
Adolfsson, J., et al.: Upregulation of Flt3 expression within the bone marrow Lin(−)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 15(4), 659–669 (2001)
Nilsson, S.K., et al.: Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 106(4), 1232–1239 (2005)
Coombe, D.R., Kett, W.C.: Heparan sulfate-protein interactions: therapeutic potential through structure-function insights. Cell. Mol. Life Sci. 62(4), 410–424 (2005)
Han, Z.C., et al.: Glycosaminoglycans enhance megakaryocytopoiesis by modifying the activities of hematopoietic growth regulators. J. Cell. Physiol. 168(1), 97–104 (1996)
Gupta, P., McCarthy, J.B., Verfaillie, C.M.: Stromal fibroblast heparan sulfate is required for cytokine-mediated ex vivo maintenance of human long-term culture-initiating cells. Blood. 87(8), 3229–3236 (1996)
Verfaillie, C.M., Miller, J.S.: CD34+/CD33- cells reselected from macrophage inflammatory protein 1 alpha+interleukin-3--supplemented "stroma-noncontact" cultures are highly enriched for long-term bone marrow culture initiating cells. Blood. 84(5), 1442–1449 (1994)
Hoogewerf, A.J., et al.: Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry. 36(44), 13570–13578 (1997)
Xu, Y., Liu, Y.J., Yu, Q.: Angiopoietin-3 is tethered on the cell surface via heparan sulfate proteoglycans. J. Biol. Chem. 279(39), 41179–41188 (2004)
Cazes, A., et al.: Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial cell adhesion, migration, and sprouting and alters actin cytoskeleton. Circ. Res. 99(11), 1207–1215 (2006)
Netelenbos, T., et al.: Proteoglycans on bone marrow endothelial cells bind and present SDF-1 towards hematopoietic progenitor cells. Leukemia. 17(1), 175–184 (2003)
Dar, A., Kollet, O., Lapidot, T.: Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp. Hematol. 34(8), 967–975 (2006)
Bhardwaj, G., et al.: Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat. Immunol. 2(2), 172–180 (2001)
Bhatia, M., et al.: Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells. J. Exp. Med. 189(7), 1139–1148 (1999)
Khan, S.A., et al.: Endogenous heparan sulfate and heparin modulate bone morphogenetic protein-4 signaling and activity. Am. J. Physiol. Cell. Physiol. 294(6), C1387–C1397 (2008)
Jackson, R.A., Nurcombe, V., Cool, S.M.: Coordinated fibroblast growth factor and heparan sulfate regulation of osteogenesis. Gene. 379, 79–91 (2006)
Yeoh, J.S., et al.: Fibroblast growth factor-1 and -2 preserve long-term repopulating ability of hematopoietic stem cells in serum-free cultures. Stem Cells. 24(6), 1564–1572 (2006)
Marie, P.J., Coffin, J.D., Hurley, M.M.: FGF and FGFR signaling in chondrodysplasias and craniosynostosis. J. Cell. Biochem. 96(5), 888–896 (2005)
Bonaventure, J., El Ghouzzi, V.: Molecular and cellular bases of syndromic craniosynostoses. Expert Rev. Mol. Med. 5(4), 1–17 (2003)
Marie, P.J.: Fibroblast growth factor signaling controlling osteoblast differentiation. Gene. 316, 23–32 (2003)
Turnbull, J.E., et al.: Identification of the basic fibroblast growth factor binding sequence in fibroblast heparan sulfate. J. Biol. Chem. 267(15), 10337–10341 (1992)
Pellegrini, L., et al.: Crystal structure of fibroblast growth factor receptor ectodomain bound to ligand and heparin. Nature. 407(6807), 1029–1034 (2000)
Jackson, R.A., et al.: Heparan sulfate regulates the anabolic activity of MC3T3-E1 preosteoblast cells by induction of Runx2. J. Cell. Physiol. 210(1), 38–50 (2007)
Logan, C.Y., Nusse, R.: The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 (2004)
Bejsovec, A.: Wnt pathway activation: new relations and locations. Cell. 120(1), 11–14 (2005)
Morimoto-Tomita, M., et al.: Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J. Biol. Chem. 277(51), 49175–49185 (2002)
Hoppler, S., Kavanagh, C.L.: Wnt signalling: variety at the core. J. Cell Sci. 120(Pt 3), 385–393 (2007)
Anton, R., Kestler, H.A., Kuhl, M.: Beta-catenin signaling contributes to stemness and regulates early differentiation in murine embryonic stem cells. FEBS Lett. 581(27), 5247–5254 (2007)
Duncan, A.W., et al.: Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat. Immunol. 6(3), 314–322 (2005)
Lai, X.N., et al.: Sensory neuropeptide SP modulating expression of EGF, FGF-2 and their receptors in fibroblasts of granulation in vivo and in vitro. Zhonghua Yi Xue Za Zhi. 83(16), 1433–1436 (2003)
Schofield, K.P., Gallagher, J.T., David, G.: Expression of proteoglycan core proteins in human bone marrow stroma. Biochem. J. 343(Pt 3), 663–668 (1999)
Spooncer, E., et al.: Regulation of haemopoiesis in long-term bone marrow cultures. IV. Glycosaminoglycan synthesis and the stimulation of haemopoiesis by beta-D-xylosides. J. Cell Biol. 96(2), 510–514 (1983)
Spooncer, E., Dexter, T.M.: Transplantation of long-term cultured bone marrow cells. Transplantation. 35(6), 624–627 (1983)
Gallagher, J.T.: E. Spooncer, and T.M. Dexter, Role of the cellular matrix in haemopoiesis. I. Synthesis of glycosaminoglycans by mouse bone marrow cell cultures. J. Cell Sci. 63, 155–171 (1983)
Wight, T.N., et al.: Proteoglycans in human long-term bone marrow cultures: biochemical and ultrastructural analyses. Blood. 67(5), 1333–1343 (1986)
Gallagher, J.T., Lyon, M., Steward, W.P.: Structure and function of heparan sulphate proteoglycans. Biochem. J. 236(2), 313–325 (1986)
Drzeniek, Z., et al.: Proteoglycan synthesis in haematopoietic cells: isolation and characterization of heparan sulphate proteoglycans expressed by the bone-marrow stromal cell line MS-5. Biochem. J. 327(Pt 2), 473–480 (1997)
Gupta, P., et al.: Structurally specific heparan sulfates support primitive human hematopoiesis by formation of a multimolecular stem cell niche. Blood. 92(12), 4641–4651 (1998)
Rodgers, K.D., San Antonio, J.D., Jacenko, O.: Heparan sulfate proteoglycans: a GAGgle of skeletal-hematopoietic regulators. Dev. Dyn. 237(10), 2622–2642 (2008)
Bruno, E., et al.: Marrow-derived heparan sulfate proteoglycan mediates the adhesion of hematopoietic progenitor cells to cytokines. Exp. Hematol. 23(11), 1212–1217 (1995)
Siczkowski, M., Clarke, D., Gordon, M.Y.: Binding of primitive hematopoietic progenitor cells to marrow stromal cells involves heparan sulfate. Blood. 80(4), 912–919 (1992)
Wight, T.N., Kinsella, M.G., Qwarnstrom, E.E.: The role of proteoglycans in cell adhesion, migration and proliferation. Curr. Opin. Cell Biol. 4(5), 793–801 (1992)
Bernfield, M., et al.: Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu. Rev. Cell Biol. 8, 365–393 (1992)
Chen, X.D., et al.: Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J. Bone Miner. Res. 22(12), 1943–1956 (2007)
Tumova, S., Woods, A., Couchman, J.R.: Heparan sulfate proteoglycans on the cell surface: versatile coordinators of cellular functions. Int. J. Biochem. Cell Biol. 32(3), 269–288 (2000)
Rapraeger, A.C.: Syndecan-regulated receptor signaling. J. Cell Biol. 149(5), 995–998 (2000)
Zaragosi, L.E., et al.: Syndecan-1 regulates adipogenesis: new insights in dedifferentiated liposarcoma tumorigenesis. Carcinogenesis. 36(1), 32–40 (2014)
Gotte, M., et al.: Role of syndecan-1 in leukocyte-endothelial interactions in the ocular vasculature. Invest. Ophthalmol. Vis. Sci. 43(4), 1135–1141 (2002)
Andersen, N.F., et al.: Upregulation of Syndecan-1 in the bone marrow microenvironment in multiple myeloma is associated with angiogenesis. Eur. J. Haematol. 95(3), 211–217 (2015)
Mahtouk, K., et al.: Heparanase influences expression and shedding of syndecan-1, and its expression by the bone marrow environment is a bad prognostic factor in multiple myeloma. Blood. 109(11), 4914–4923 (2007)
Jung, O., et al.: Heparanase-induced shedding of syndecan-1/CD138 in myeloma and endothelial cells activates VEGFR2 and an invasive phenotype: prevention by novel synstatins. Oncogene. 5, e202 (2016)
Filmus, J.: Glypicans in growth control and cancer. Glycobiology. 11(3), 19R–23R (2001)
Yanagishita, M., Hascall, V.C.: Cell surface heparan sulfate proteoglycans. J. Biol. Chem. 267(14), 9451–9454 (1992)
Viviano, B.L., et al.: Altered hematopoiesis in glypican-3-deficient mice results in decreased osteoclast differentiation and a delay in endochondral ossification. Dev. Biol. 282(1), 152–162 (2005)
Haupt, L.M., et al.: The heparan sulfate proteoglycan (HSPG) glypican-3 mediates commitment of MC3T3-E1 cells toward osteogenesis. J. Cell. Physiol. 220(3), 780–791 (2009)
Teplyuk, N.M., et al.: The osteogenic transcription factor Runx2 regulates components of the fibroblast growth factor/proteoglycan signaling axis in osteoblasts. J. Cell. Biochem. 107(1), 144–154 (2009)
Khurana, S., et al.: Glypican-3-mediated inhibition of CD26 by TFPI: a novel mechanism in hematopoietic stem cell homing and maintenance. Blood. 121(14), 2587–2595 (2013)
Litwack, E.D., et al.: Expression of the heparan sulfate proteoglycan glypican-1 in the developing rodent. Dev. Dyn. 211(1), 72–87 (1998)
Siebertz, B., et al.: Expression of glypican-4 in haematopoietic-progenitor and bone-marrow-stromal cells. Biochem. J. 344(Pt 3), 937–943 (1999)
Arikawa-Hirasawa, E., et al.: Perlecan is essential for cartilage and cephalic development. Nat. Genet. 23(3), 354–358 (1999)
Thompson, W.R., et al.: Perlecan/Hspg2 deficiency alters the pericellular space of the lacunocanalicular system surrounding osteocytic processes in cortical bone. J. Bone Miner. Res. 26(3), 618–629 (2011)
Wang, B., et al.: Perlecan-containing pericellular matrix regulates solute transport and mechanosensing within the osteocyte lacunar-canalicular system. J. Bone Miner. Res. 29(4), 878–891 (2014)
Gustafsson, E., et al.: Role of collagen type II and perlecan in skeletal development. Ann. N. Y. Acad. Sci. 995, 140–150 (2003)
Ishijima, M., et al.: Perlecan modulates VEGF signaling and is essential for vascularization in endochondral bone formation. Matrix Biol. 31(4), 234–245 (2012)
Klein, G., et al.: Perlecan in human bone marrow: a growth-factor-presenting, but anti-adhesive, extracellular matrix component for hematopoietic cells. Matrix Biol. 14(6), 457–465 (1995)
Yang, W.D., et al.: Perlecan domain I promotes fibroblast growth factor 2 delivery in collagen I fibril scaffolds. Tissue Eng. 11(1–2), 76–89 (2005)
Muthusamy, A., Cooper, C.R., Gomes Jr., R.R.: Soluble perlecan domain I enhances vascular endothelial growth factor-165 activity and receptor phosphorylation in human bone marrow endothelial cells. BMC Biochem. 11, 43 (2010)
Lai, Y., et al.: Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells. Stem Cells Dev. 19(7), 1095–1107 (2010)
Nigro, J., et al.: The effect of bovine endosteum-derived particles on the proliferation of human mesenchymal stem cells. Biomaterials. 31(21), 5689–5699 (2010)
Nakamura, R., Nakamura, F., Fukunaga, S.: Contrasting effect of perlecan on adipogenic and osteogenic differentiation of mesenchymal stem cells in vitro. Anim. Sci. J. 85(3), 262–270 (2014)
Decarlo, A.A., et al.: Perlecan domain 1 recombinant proteoglycan augments BMP-2 activity and osteogenesis. BMC Biotechnol. 12, 60 (2012)
Ruegg, M.A., Bixby, J.L.: Agrin orchestrates synaptic differentiation at the vertebrate neuromuscular junction. Trends Neurosci. 21(1), 22–27 (1998)
Kim, N., et al.: Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 135(2), 334–342 (2008)
Hausser, H.J., et al.: Agrin is highly expressed by chondrocytes and is required for normal growth. Histochem. Cell Biol. 127(4), 363–374 (2007)
Mazzon, C., et al.: Agrin is required for survival and function of monocytic cells. Blood. 119(23), 5502–5511 (2012)
Zhang, J., et al.: Agrin is involved in lymphocytes activation that is mediated by alpha-dystroglycan. FASEB J. 20(1), 50–58 (2006)
Barresi, R., Campbell, K.P.: Dystroglycan: from biosynthesis to pathogenesis of human disease. J. Cell Sci. 119(Pt 2), 199–207 (2006)
Yang, B., et al.: SH3 domain-mediated interaction of dystroglycan and Grb2. J. Biol. Chem. 270(20), 11711–11714 (1995)
Koch, C.A., et al.: SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 252(5006), 668–674 (1991)
Steidl, U., et al.: Primary human CD34+ hematopoietic stem and progenitor cells express functionally active receptors of neuromediators. Blood. 104(1), 81–88 (2004)
Mazzon, C., et al.: The critical role of agrin in the hematopoietic stem cell niche. Blood. 118(10), 2733–2742 (2011)
Kiel, M.J., et al.: SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 121(7), 1109–1121 (2005)
To, L.B, et al.: The biology and clinical uses of blood stem cells. Blood. 89(7), 2233–2258 (1997)
Weaver, C.H., Schulman, K.A., Buckner, C.D.: Mobilization of peripheral blood stem cells following myelosuppressive chemotherapy: a randomized comparison of filgrastim, sargramostim, or sequential sargramostim and filgrastim. Bone Marrow Transplant. 27(Suppl 2), S23–S29 (2001)
Winkler, I.G., Levesque, J.P.: Mechanisms of hematopoietic stem cell mobilization: when innate immunity assails the cells that make blood and bone. Exp. Hematol. 34(8), 996–1009 (2006)
Ma, Q., et al.: Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 95(16), 9448–9453 (1998)
Nagasawa, T., et al.: Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 382(6592), 635–638 (1996)
Bleul, C.C., et al.: A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J. Exp. Med. 184(3), 1101–1109 (1996)
Netelenbos, T., et al.: Proteoglycans guide SDF-1-induced migration of hematopoietic progenitor cells. J. Leukoc. Biol. 72(2), 353–362 (2002)
Avigdor, A., et al.: CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 103(8), 2981–2989 (2004)
Amara, A., et al.: Stromal cell-derived factor-1alpha associates with heparan sulfates through the first beta-strand of the chemokine. J. Biol. Chem. 274(34), 23916–23925 (1999)
Sadir, R., et al.: Characterization of the stromal cell-derived factor-1alpha-heparin complex. J. Biol. Chem. 276(11), 8288–8296 (2001)
Frenette, P.S., Weiss, L.: Sulfated glycans induce rapid hematopoietic progenitor cell mobilization: evidence for selectin-dependent and independent mechanisms. Blood. 96(7), 2460–2468 (2000)
Sweeney, E.A., et al.: Sulfated polysaccharides increase plasma levels of SDF-1 in monkeys and mice: involvement in mobilization of stem/progenitor cells. Blood. 99(1), 44–51 (2002)
Albanese, P., et al.: Glycosaminoglycan mimetics-induced mobilization of hematopoietic progenitors and stem cells into mouse peripheral blood: structure/function insights. Exp. Hematol. 37(9), 1072–1083 (2009)
Papy-Garcia, D., et al.: Nondegradative sulfation of polysaccharides. Synthesis and structure characterization of biologically active heparan sulfate mimetics. Macromolecules. 38, 4647–4654 (2005)
Saez, B., et al.: Inhibiting stromal cell heparan sulfate synthesis improves stem cell mobilization and enables engraftment without cytotoxic conditioning. Blood. 124(19), 2937–2947 (2014)
Pittenger, M.F., et al.: Multilineage potential of adult human mesenchymal stem cells. Science. 284(5411), 143–147 (1999)
Muschler, G.F., Nakamoto, C., Griffith, L.G.: Engineering principles of clinical cell-based tissue engineering. J. Bone Joint Surg. Am. 86-A(7), 1541–1558 (2004)
Wexler, S.A., et al.: Adult bone marrow is a rich source of human mesenchymal 'stem' cells but umbilical cord and mobilized adult blood are not. Br. J. Haematol. 121(2), 368–374 (2003)
Baksh, D., Song, L., Tuan, R.S.: Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J. Cell. Mol. Med. 8(3), 301–316 (2004)
Dombrowski, C., et al.: Heparan sulfate mediates the proliferation and differentiation of rat mesenchymal stem cells. Stem Cells Dev. 18(4), 661–670 (2009)
Manton, K.J., et al.: Disruption of heparan and chondroitin sulfate signaling enhances mesenchymal stem cell-derived osteogenic differentiation via bone morphogenetic protein signaling pathways. Stem Cells. 25(11), 2845–2854 (2007)
Cool, S.M., Nurcombe, V.: The osteoblast-heparan sulfate axis: control of the bone cell lineage. Int. J. Biochem. Cell Biol. 37(9), 1739–1745 (2005)
Nurcombe, V., et al.: Temporal and functional changes in glycosaminoglycan expression during osteogenesis. J. Mol. Histol. 38(5), 469–481 (2007)
Montero, A., et al.: Disruption of the fibroblast growth factor-2 gene results in decreased bone mass and bone formation. J. Clin. Invest. 105(8), 1085–1093 (2000)
Muller, B., et al.: Increased levels of xylosyltransferase I correlate with the mineralization of the extracellular matrix during osteogenic differentiation of mesenchymal stem cells. Matrix Biol. 27(2), 139–149 (2008)
Molteni, A., et al.: Alterations of matrix- and cell-associated proteoglycans inhibit osteogenesis and growth response to fibroblast growth factor-2 in cultured rat mandibular condyle and calvaria. Cell Tissue Res. 295(3), 523–536 (1999)
Ostrovsky, O., et al.: Differential effects of heparin saccharides on the formation of specific fibroblast growth factor (FGF) and FGF receptor complexes. J. Biol. Chem. 277(4), 2444–2453 (2002)
Goodger, S.J., et al.: Evidence that heparin saccharides promote FGF2 mitogenesis through two distinct mechanisms. J. Biol. Chem. 283(19), 13001–13008 (2008)
Guimond, S.E., Turnbull, J.E.: Fibroblast growth factor receptor signalling is dictated by specific heparan sulphate saccharides. Curr. Biol. 9(22), 1343–1346 (1999)
Frescaline, G., et al.: Glycosaminoglycans mimetics potentiate the clonogenicity, proliferation, migration and differentiation properties of rat mesenchymal stem cells. Stem Cell Res. 8(2), 180–192 (2012)
Frescaline, G., et al.: Glycosaminoglycan mimetic associated to human mesenchymal stem cell-based scaffolds inhibit ectopic bone formation, but induce angiogenesis in vivo. Tissue Eng. Part A. 19(13–14), 1641–1653 (2013)
Grellier, M., Bordenave, L., Amedee, J.: Cell-to-cell communication between osteogenic and endothelial lineages: implications for tissue engineering. Trends Biotechnol. 27(10), 562–571 (2009)
Esko, J.D., Lindahl, U.: Molecular diversity of heparan sulfate. J. Clin. Invest. 108(2), 169–173 (2001)
Kusche-Gullberg, M., et al.: Identification and expression in mouse of two heparan sulfate glucosaminyl N-deacetylase/N-sulfotransferase genes. J. Biol. Chem. 273(19), 11902–11907 (1998)
Grobe, K., et al.: Cerebral hypoplasia and craniofacial defects in mice lacking heparan sulfate Ndst1 gene function. Development. 132(16), 3777–3786 (2005)
Fan, G., et al.: Targeted disruption of NDST-1 gene leads to pulmonary hypoplasia and neonatal respiratory distress in mice. FEBS Lett. 467(1), 7–11 (2000)
Ringvall, M., et al.: Defective heparan sulfate biosynthesis and neonatal lethality in mice lacking N-deacetylase/N-sulfotransferase-1. J. Biol. Chem. 275(34), 25926–25930 (2000)
Forsberg, E., et al.: Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 400(6746), 773–776 (1999)
Humphries, D.E., et al.: Heparin is essential for the storage of specific granule proteases in mast cells. Nature. 400(6746), 769–772 (1999)
Pallerla, S.R., et al.: Altered heparan sulfate structure in mice with deleted NDST3 gene function. J. Biol. Chem. 283(24), 16885–16894 (2008)
Jakobsson, L., et al.: Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis. Dev. Cell. 10(5), 625–634 (2006)
Forsberg, M., et al.: Undersulfation of heparan sulfate restricts differentiation potential of mouse embryonic stem cells. J. Biol. Chem. 287(14), 10853–10862 (2012)
Zhao, S., et al.: Heparan sulfate 6-O-sulfotransferase 3 is involved in bone marrow mesenchymal stromal cell osteogenic differentiation. Biochemistry (Mosc). 80(3), 379–389 (2015)
Shukla, D., et al.: A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 99(1), 13–22 (1999)
Patel, V.N., et al.: Hs3st3-modified heparan sulfate controls KIT+ progenitor expansion by regulating 3-O-sulfotransferases. Dev. Cell. 29(6), 662–673 (2014)
Hirano, K., Van Kuppevelt, T.H., Nishihara, S.: The transition of mouse pluripotent stem cells from the naive to the primed state requires Fas signaling through 3-O sulfated heparan sulfate structures recognized by the HS4C3 antibody. Biochem. Biophys. Res. Commun. 430(3), 1175–1181 (2013)
Hirano, K., et al.: 3-O-sulfated heparan sulfate recognized by the antibody HS4C3 contributes [corrected] to the differentiation of mouse embryonic stem cells via fas signaling. PLoS One. 7(8), e43440 (2012)
Martinez, P., et al.: Macrophage polarization alters the expression and sulfation pattern of glycosaminoglycans. Glycobiology. 25(5), 502–513 (2015)
Sikora, A.S., et al.: Regulation of the expression of Heparan sulfate 3-O-sulfotransferase 3B (HS3ST3B) by inflammatory stimuli in human monocytes. J. Cell. Biochem. 117(7), 1529–1542 (2016)
Kram, V., et al.: Heparanase is expressed in osteoblastic cells and stimulates bone formation and bone mass. J. Cell. Physiol. 207(3), 784–792 (2006)
Schmidt, B., et al.: A novel amino acid modification in sulfatases that is defective in multiple sulfatase deficiency. Cell. 82(2), 271–278 (1995)
Cosma, M.P., et al.: The multiple sulfatase deficiency gene encodes an essential and limiting factor for the activity of sulfatases. Cell. 113(4), 445–456 (2003)
Dierks, T., et al.: Multiple sulfatase deficiency is caused by mutations in the gene encoding the human C(alpha)-formylglycine generating enzyme. Cell. 113(4), 435–444 (2003)
Sardiello, M., et al.: Sulfatases and sulfatase modifying factors: an exclusive and promiscuous relationship. Hum. Mol. Genet. 14(21), 3203–3217 (2005)
Cosma, M.P., et al.: Molecular and functional analysis of SUMF1 mutations in multiple sulfatase deficiency. Hum. Mutat. 23(6), 576–581 (2004)
Settembre, C., et al.: Systemic inflammation and neurodegeneration in a mouse model of multiple sulfatase deficiency. Proc. Natl. Acad. Sci. U. S. A. 104(11), 4506–4511 (2007)
Buono, M., et al.: Sulfatase modifying factor 1-mediated fibroblast growth factor signaling primes hematopoietic multilineage development. J. Exp. Med. 207(8), 1647–1660 (2010)
Taichman, R.S.: Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 105(7), 2631–2639 (2005)
Morrison, S.J., Spradling, A.C.: Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 132(4), 598–611 (2008)
Bianco, P., et al.: Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 19(3), 180–192 (2001)
Katayama, Y., et al.: Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 124(2), 407–421 (2006)
Gordon, M.Y.: Extracellular matrix of the marrow microenvironment. Br. J. Haematol. 70(1), 1–4 (1988)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Papy-Garcia, D., Albanese, P. Heparan sulfate proteoglycans as key regulators of the mesenchymal niche of hematopoietic stem cells. Glycoconj J 34, 377–391 (2017). https://doi.org/10.1007/s10719-017-9773-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-017-9773-8