Abstract
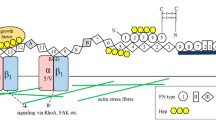
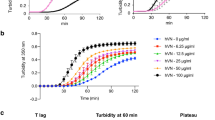
Vitronectin (VN) plays an important role in tissue regeneration. We previously reported that VN from partial hepatectomized (PH) rats results in a decrease of sialylation of VN and de-sialylation of VN decreases the cell spreading of hepatic stellate cells. In this study, we analyzed the mechanism how sialylation of VN regulates the properties of mouse primary cultured dermal fibroblasts (MDF) and a dermal fibroblast cell line, Swiss 3T3 cells. At first, we confirmed that VN from PH rats or de-sialylated VN also decreased cell spreading in MDF and Swiss 3T3 cells. The de-sialylation suppressed stress fiber formation in Swiss 3T3 cells. Next, we analyzed the effect of the de-sialylation of VN on stress fiber formation in Swiss 3T3 cells. RGD peptide, an inhibitor for a cell binding site of VN, did not affect the cell attachment of Swiss 3T3 cells on untreated VN but significantly decreased it on de-sialylated VN, suggesting that the de-sialylation attenuates the binding activity of an RGD-independent binding site in VN. To analyze a candidate RGD-independent binding site, an inhibition experiment of stress fiber formation for a heparin binding site was performed. The addition of heparin and treatment of cells with heparinase decreased stress fiber formation in Swiss 3T3 cells. Furthermore, de-sialylation increased the binding activity of VN to heparin, as detected by surface plasmon resonance (SPR). These results demonstrate that sialylation of VN glycans regulates stress fiber formation and cell spreading of dermal fibroblast cells via a heparin binding site.







Similar content being viewed by others
Abbreviations
- Con A:
-
Concanavalin A
- ECM:
-
Extracellular matrix
- FAK:
-
Focal adhesion kinase
- HRP:
-
Horseradish peroxidase
- MDF:
-
Mouse dermal fibroblast
- NO:
-
Non-operated
- PAI-1:
-
Plasminogen activator inhibitor-1
- PH:
-
Partially hepatectomized
- PNGase F:
-
Peptide-N4-(N-acetyl-β-D-glucosaminyl) asparagine amidase from Fravobacterium meningosepticum
- SO:
-
Sham-operated
- SNA:
-
Sambucus nigra agglutinin
- SPR:
-
Surface plasmon resonance
- VN:
-
Vitronectin
References
Preissner K.T., Reuning U.: Vitronectin in vascular context: facets of a multitalented matricellular protein. Semin Thromb Hemost. 37(4), 408–424 (2011)
Leavesley D.I., Kashyap A.S., Croll T., Sivaramakrishnan M., Shokoohmand A., Hollier B.G., Upton Z.: Vitronectin–master controller or micromanager? IUBMB Life. 65(10), 807–818 (2013)
Preissner K.T.: Structure and biological role of vitronectin. Annu Rev Cell Biol. 7, 275–310 (1991)
Schvartz I., Seger D., Shaltiel S.: Vitronectin Int J Biochem Cell Biol. 31(5), 539–544 (1999)
Fay W.P., Parker A.C., Ansari M.N., Zheng X., Ginsburg D.: Vitronectin inhibits the thrombotic response to arterial injury in mice. Blood. 93(6), 1825–1830 (1999)
Wang A.G., Yen M.Y., Hsu W.M., Fann M.J.: Induction of vitronectin and integrin alphav in the retina after optic nerve injury. Mol Vis. 12, 76–84 (2006)
Li R., Luo M., Ren M., Chen N., Xia J., Deng X., Zeng M., Yan K., Luo T., Wu J.: Vitronectin regulation of vascular endothelial growth factor-mediated angiogenesis. J Vasc Res. 51(2), 110–117 (2014)
Seiffert D.: Evidence that conformational changes upon the transition of the native to the modified form of vitronectin are not limited to the heparin binding domain. FEBS Lett. 368(1), 155–159 (1995)
Seiffert D., Loskutoff D.J.: Type 1 plasminogen activator inhibitor induces multimerization of plasma vitronectin. A suggested mechanism for the generation of the tissue form of vitronectin in vivo. J Biol Chem. 271(47), 29644–29651 (1996)
Izumi M., Yamada K.M., Hayashi M.: Vitronectin exists in two structurally and functionally distinct forms in human plasma. Biochim Biophys Acta. 990(2), 101–108 (1989)
Stockmann A., Hess S., Declerck P., Timpl R., Preissner K.T.: Multimeric vitronectin. Identification and characterization of conformation-dependent self-association of the adhesive protein. J Biol Chem. 268(30), 22874–22882 (1993)
Preissner K.T., Muller-Berghaus G.: Neutralization and binding of heparin by S protein/vitronectin in the inhibition of factor Xa by antithrombin III. Involvement of an inducible heparin-binding domain of S protein/vitronectin. J. Biol. Chem. 262(25), 12247–12253 (1987)
Seiffert D.: The glycosaminoglycan binding site governs ligand binding to the somatomedin B domain of vitronectin. J. Biol. Chem. 272(15), 9971–9978 (1997)
Ogawa H., Sano K., Sobukawa N., Asanuma-Date K.: “Matrix Restructuring During Liver Regeneration is Regulated by Glycosylation of the Matrix Glycoprotein Vitronectin”. In: Baptista P. (ed.) “Liver regeneration, pp. 79–98. InTech Publishers,, Open access (2012)
Dufourcq P., Couffinhal T., Alzieu P., Daret D., Moreau C., Duplaa C., Bonnet J.: Vitronectin is up-regulated after vascular injury and vitronectin blockade prevents neointima formation. Cardiovasc Res. 53(4), 952–962 (2002)
Sano K., Miyamoto Y., Kawasaki N., Hashii N., Itoh S., Murase M., Date K., Yokoyama M., Sato C., Kitajima K., Ogawa H.: Survival signals of hepatic stellate cells in liver regeneration are regulated by glycosylation changes in rat vitronectin, especially decreased sialylation. J Biol Chem. 285(23), 17301–17309 (2010)
Uchibori-Iwaki H., Yoneda A., Oda-Tamai S., Kato S., Akamatsu N., Otsuka M., Murase K., Kojima K., Suzuki R., Maeya Y., Tanabe M., Ogawa H.: The changes in glycosylation after partial hepatectomy enhance collagen binding of vitronectin in plasma. Glycobiology. 10(9), 865–874 (2000)
Yasukawa Z., Sato C., Sano K., Ogawa H., Kitajima K.: Identification of disialic acid-containing glycoproteins in mouse serum: a novel modification of immunoglobulin light chains, vitronectin, and plasminogen. Glycobiology. 16(7), 651–665 (2006)
Yatohgo T., Izumi M., Kashiwagi H., Hayashi M.: Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 13(4), 281–292 (1988)
Ueda H., Kojima K., Saitoh T., Ogawa H.: Interaction of a lectin from Psathyrella velutina mushroom with N-acetylneuraminic acid. FEBS Lett. 448(1), 75–80 (1999)
Yoneda A., Ogawa H., Kojima K., Matsumoto I.: Characterization of the ligand binding activities of vitronectin: interaction of vitronectin with lipids and identification of the binding domains for various ligands using recombinant domains. Biochemistry. 37(18), 6351–6360 (1998)
Fischer S.M., Viaje A., Mills G.D., Slaga T.J.: Explant methods for epidermal cell culture. Methods Cell Biol. 21A, 207–227 (1980)
Takekawa H., Ina C., Sato R., Toma K., Ogawa H.: Novel carbohydrate-binding activity of pancreatic trypsins to N-linked glycans of glycoproteins. J Biol Chem. 281(13), 8528–8538 (2006)
Osmond R.I., Kett W.C., Skett S.E., Coombe D.R.: Protein-heparin interactions measured by BIAcore 2000 are affected by the method of heparin immobilization. Anal Biochem. 310(2), 199–207 (2002)
Nakagawa K., Nakamura K., Haishima Y., Yamagami M., Saito K., Sakagami H., Ogawa H.: Pseudoproteoglycan (pseudoPG) probes that simulate PG macromolecular structure for screening and isolation of PG-binding proteins. Glycoconj J. 26(8), 1007–1017 (2009)
Yoneda A., Ogawa H., Matsumoto I., Ishizuka I., Hase S., Seno N.: Structures of the N-linked oligosaccharides on porcine plasma vitronectin. Eur J Biochem. 218(3), 797–806 (1993)
Jurjus R.A., Liu Y., Pal-Ghosh S., Tadvalkar G., Stepp M.A.: Primary dermal fibroblasts derived from sdc-1 deficient mice migrate faster and have altered alphav integrin function. Wound Repair Regen. 16(5), 649–660 (2008)
Bass M.D., Williamson R.C., Nunan R.D., Humphries J.D., Byron A., Morgan M.R., Martin P., Humphries M.J.: A syndecan-4 hair trigger initiates wound healing through caveolin- and RhoG-regulated integrin endocytosis. Dev Cell. 21(4), 681–693 (2011)
Granes F., Garcia R., Casaroli-Marano R.P., Castel S., Rocamora N., Reina M., Urena J.M., Vilaro S.: Syndecan-2 induces filopodia by active cdc42Hs. Exp Cell Res. 248(2), 439–456 (1999)
Gailit J., Clark R.A.: Studies in vitro on the role of alpha v and beta 1 integrins in the adhesion of human dermal fibroblasts to provisional matrix proteins fibronectin, vitronectin, and fibrinogen. J Investig Dermatol. 106(1), 102–108 (1996)
Reynolds L.E., Conti F.J., Lucas M., Grose R., Robinson S., Stone M., Saunders G., Dickson C., Hynes R.O., Lacy-Hulbert A., Hodivala-Dilke K.: Accelerated re-epithelialization in beta3-integrin-deficient- mice is associated with enhanced TGF-beta1 signaling. Nat Med. 11(2), 167–174 (2005)
Sano K., Asanuma-Date K., Arisaka F., Hattori S., Ogawa H.: Changes in glycosylation of vitronectin modulate multimerization and collagen binding during liver regeneration. Glycobiology. 17(7), 784–794 (2007)
Naski M.C., Lawrence D.A., Mosher D.F., Podor T.J., Ginsburg D.: Kinetics of inactivation of alpha-thrombin by plasminogen activator inhibitor-1. Comparison of the effects of native and urea-treated forms of vitronectin. J. Biol. Chem. 268(17), 12367–12372 (1993)
Tschopp J., Masson D., Schafer S., Peitsch M., Preissner K.T.: The heparin binding domain of S-protein/vitronectin binds to complement components C7, C8, and C9 and perforin from cytolytic T-cells and inhibits their lytic activities. Biochemistry. 27(11), 4103–4109 (1988)
Wilkins-Port C.E., Sanderson R.D., Tominna-Sebald E., McKeown-Longo P.J.: Vitronectin's basic domain is a syndecan ligand which functions in trans to regulate vitronectin turnover. Cell Commun Adhes. 10(2), 85–103 (2003)
Wilkins-Port C.E., McKeown-Longo P.J.: Heparan sulfate proteoglycans function in the binding and degradation of vitronectin by fibroblast monolayers. Biochem Cell Biol. 74(6), 887–897 (1996)
Echtermeyer F., Baciu P.C., Saoncella S., Ge Y., Goetinck P.F.: Syndecan-4 core protein is sufficient for the assembly of focal adhesions and actin stress fibers. J Cell Sci. 112(Pt 20), 3433–3441 (1999)
Dovas A., Yoneda A., Couchman J.R.: PKCbeta-dependent activation of RhoA by syndecan-4 during focal adhesion formation. J Cell Sci. 119(Pt 13), 2837–2846 (2006)
Couchman J.R., Woods A.: Syndecan-4 and integrins: combinatorial signaling in cell adhesion. J Cell Sci. 112(Pt 20), 3415–3420 (1999)
Cardin A.D., Weintraub H.J.: Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 9(1), 21–32 (1989)
Lane D.A., Flynn A.M., Pejler G., Lindahl U., Choay J., Preissner K.: Structural requirements for the neutralization of heparin-like saccharides by complement S protein/vitronectin. J. Biol. Chem. 262(34), 16343–16348 (1987)
Liang O.D., Rosenblatt S., Chhatwal G.S., Preissner K.T.: Identification of novel heparin-binding domains of vitronectin. FEBS Lett. 407(2), 169–172 (1997)
Gibson A.D., Lamerdin J.A., Zhuang P., Baburaj K., Serpersu E.H., Peterson C.B.: Orientation of heparin-binding sites in native vitronectin. Analyses of ligand binding to the primary glycosaminoglycan-binding site indicate that putative secondary sites are not functional. J. Biol. Chem. 274(10), 6432–6442 (1999)
Yoneda A., Kojima K., Matsumoto I., Yamamoto K., Ogawa H.: Porcine vitronectin, the most compact form of single-chain vitronectin: the smallest molecular mass among vitronectins was ascribed to deletion and substitution of base pairs, and proteolytic trimming of the peptide. J Biochem. 120(5), 954–960 (1996)
Chillakuri C.R., Jones C., Mardon H.J.: Heparin binding domain in vitronectin is required for oligomerization and thus enhances integrin mediated cell adhesion and spreading. FEBS Lett. 584(15), 3287–3291 (2010)
Clark R.A., Tonnesen M.G., Gailit J., Cheresh D.A.: Transient functional expression of alphaVbeta 3 on vascular cells during wound repair. Am J Pathol. 148(5), 1407–1421 (1996)
Weckroth M., Vaheri A., Virolainen S., Saarialho-Kere U., Jahkola T., Siren V.: Epithelial tissue-type plasminogen activator expression, unlike that of urokinase, its receptor, and plasminogen activator inhibitor-1, is increased in chronic venous ulcers. Br J Dermatol. 151(6), 1189–1196 (2004)
Olczyk P., Komosinska-Vassev K., Winsz-Szczotka K., Kozma E.M., Wisowski G., Stojko J., Klimek K., Olczyk K.: Propolis modulates vitronectin, laminin, and heparan sulfate/heparin expression during experimental burn healing. J Zhejiang Univ Sci B. 13(11), 932–941 (2012)
Acknowledgments
We thank Dr. Atsuko Yoneda (Tokyo University of Pharmacy and Life Sciences, Tokyo, JAPAN) for valuable suggestions and critical discussion.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Fig. S1
(PDF 115 kb)
Rights and permissions
About this article
Cite this article
Miyamoto, Y., Tanabe, M., Date, K. et al. Sialylation of vitronectin regulates stress fiber formation and cell spreading of dermal fibroblasts via a heparin-binding site. Glycoconj J 33, 227–236 (2016). https://doi.org/10.1007/s10719-016-9660-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-016-9660-8