Abstract
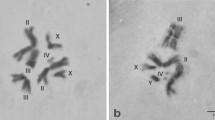
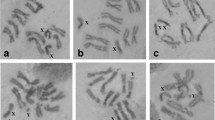
The Oriental fruit fly, Batrocera dorsalis s.s. (Hendel) is one of the most destructive agricultural pests, belonging to a large group of difficult to distinguish morphologically species, referred as the B. dorsalis complex. We report here a cytogenetic analysis of two laboratory strains of the species and provide a photographic polytene chromosome map from larval salivary glands. The mitotic complement consists of six chromosome pairs including a heteromorphic sex (XX/XY) chromosome pair. Analysis of the polytene complement has shown a total of five polytene chromosomes (10 polytene arms) that correspond to the five autosomes. The most important landmarks of each polytene chromosome and characteristic asynapsis at a specific chromosomal region are presented and discussed. Chromosomal homology between B. dorsalis and Ceratitis capitata has been determined by comparing chromosome banding patterns. The detection of chromosome inversions in both B. dorsalis strains is shown and discussed. Our results show that the polytene maps presented here are suitable for cytogenetic analysis of this species and can be used for comparative studies among species of the Tephritidae family. They also provide a diagnostic tool that could accelerate species identification within the B. dorsalis complex and could shed light on the ongoing speciation in this complex. Polytene chromosome maps can facilitate the development of biological control methods and support the genome mapping project of the species that is currently in progress.







Similar content being viewed by others
References
Adams MD, Celniker SE, Holt RA et al (2000) The genome sequence of Drosophila melanogaster. Science 287:2185–2195
Adsavakulchai A, Baimai V, Prachyabrued W, Grote PJ, Lertlum S (1998) Morphometric study using wing image analysis for identification of the Bactrocera dorsalis complex (Diptera: Tephritidae). WWW J Biol 3:34–43
Aketarawong N, Bonizzoni M, Thanaphum S, Gomulski LM, Gasperi G, Malacrida AR, Gugliemino CR (2007) Inferences on the population structure and colonization process of the invasive oriental fruit fly, Bactrocera dorsalis (Hendel). Mol Ecol 16:3522–3532
Armstrong KF, Cameron CM (2000) Species identification of tephritids across a broad taxonomic range using ribosomal DNA. In: Tan KH (ed) Area-wide control of fruit flies and other insect pests. Penerbit Universiti Sains Malaysia, Penang, pp 703–710
Armstrong KF, Cameron CM, Frampton ER (1997) Fruit fly (Diptera: Tephritidae) species identification: a rapid molecular diagnostic technique for quarantine application. Bull Entomol Res 87:111–118
Ayala FJ, Coluzzi M (2005) Chromosome speciation: Humans, Drosophila and mosquitoes. PNAS 102:6535–6542
Baimai V (1998) Heterochromatin accumulation and karyotypic evolution in some dipteran insects. Zool Stud 37:75–88
Baimai V, Trinachartvanit W, Tigvattananont S, Grote PJ, Poramarcom R, Kijchalao U (1995) Metaphase karyotypes of fruit flies of Thailand. I. Five sibling species of the Bactrocera dorsalis complex. Genome 38:1015–1022
Baimai V, Phinchongsakuldit J, Tigvattananont S (1999a) Metaphase karyotypes of fruit flies of Thailand. IV. Evidence for six new species of the Bactrocera dorsalis complex. Cytologia 64:371–377
Baimai V, Phinchongsakuldit J, Trinachartvanit W (1999b) Metaphase karyotypes of fruit flies of Thailand (III). Six members of the Bactrocera dorsalis complex. Zool Stud 38:110–118
Baimai V, Sumrandee C, Tigvattananont S, Trinachartvanit W (2000) Metaphase karyotypes of fruit flies of Thailand. V. Cytotaxonomy of ten additional new species of the Bactrocera dorsalis complex. Cytologia 65:409–417
Bedo DG (1987) Polytene chromosome mapping in Ceratitis capitata (Diptera: Tephritidae). Genome 29:598–611
Cáceres M, Ranz JM, Barbadilla A, Long M, Ruiz A (1999) A transposable element mediated the generation of a Drosophila widespread chromosomal inversion. Science 285:415–418
Cáceres M, Puig M, Ruiz A (2001) Molecular characterization of two natural hotspots in the Drosophila buzzatii genome induced by transposon insertions. Genome Res 11:1353–1364
Cáceres C, Segura DF, Vera MT, Wornoayporn W, Cladera JL, Teal P, Sapountzis P, Bourtzis K, Zacharopoulou A, Robinson AS (2009) Incipient speciation revealed in Anastrepha fraterculus by studies on mating compatibility, sex pheromones, hybridisation and cytology. Biol J Linn Soc 97:152–165
Carson HL, Yoon JS (1982) Genetics and evolution of Hawaiian Drosophila. In: Ashburner M, Carson HL, Thompson JN Jr (eds) The genetics and biology of Drosophila. Academic Press, London, 3b, pp 296–344
CDFA (California Department of Food and Agricutlure) (2008) Oriental fruit fly. Pest detection/Emergency projects branch. http://www.cdfa.ca.gov/phpps/pdep/treatment/oriental_ff.html
Chen P, Ye H (2008) Relationship among five populations of Bactrocera dorsalis based on mitochondrial DNA sequences in western Yunnan. China J Appl Entomol 132:530–537
Clarke AR, Armstrong KF, Carmichel AE, Milne JR, Raghu S, Roderick GK, Yeates DK (2005) Invasive phytophagous pest arising through a recent tropical evolutionary radiation: the Bactrocera dorsalis complex of fruit flies. Annu Rev Entomol 50:293–319
Coluzzi M, Sabatini A, Petrarca V, DiDeco MA (1979) Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg 73:483–497
Costantini C, Ayala D, Guelbeogo WM, Pombi M, Some CY, Bassole IHN, Ose K, Fotsing J-M, Sagnon N, Fontenille D, Besansky NJ, Simard F (2009) Living at the edge: biogeographic patterns of habitat segregation conform to speciation by niche expansion in Anopheles gambiae. BMC Ecol 9:16
Coyne JA, Orr HA (2004) Speciation. Sinauer Asociates, Sunderland MA
della Torre A, Fanello C, Akogbeto M, Dossou-yovo J, Favia G, Petrarca V, Coluzzi M (2001) Molecular evidence of incipient speciation within Anopheles gambiae s.s. in West Africa. Insect Mol Biol 10:9–18
Dobzhansky TG (1970) Genetics of the evolutionary process. Columbia University Press, New York
Drew RAI (1989) The tropical fruit flies (Diptera: Tephritidae: Dacinae) of the Australasian and Oceanian regions. Mem Qld Mus 26:521
Drew RAI, Hancock DL (1994) The Bactocera dorsalis complex of fruit flies (Diptera: Tephritidae: Dacinae) in Asia. Bull Entomol Res Suppl 2:1–68
Drew RAI, Raghu S, Halcoop P (2008) Bridging the morphological and biological species concepts: studies on the Bactrocera dorsalis (Hendel) complex (Diptera: Tephritidae: Dacinae) in South-East Asia. Biol J Linn Soc 93:217–226
Dunning Hotopp JC, Clark ME, Oliveira DCSG, Foster JM, Fisher P, Muñoz Torres MC, Giebel JD, Kumar N, Ishmael N, Wang S, Ingram J, Nene RV, Shepard J, Tomkins J, Richards S, Spiro DJ, Ghedin E, Slatko BE, Tettelin H, Werren JH (2007) Widespread lateral gene transfer form intracellular bacteria to multicellular eukaryotes. Science 317:1753–1756
Eichler EE, Sankoff F (2003) Structural dynamics of eukaryotic chromosome evolution. Science 301:793–797
Evgen’ev MB, Zelentsova H, Poluectova H, Lyozin GT, Veleikodvorskaja V, Pyatkov KI, Zhivotovsky LA, Kidwell MG (2000) Mobile elements and chromosomal evolution in the virilis group of Drosophila. PNAS 97:11337–11342
Feder JL, Roethele JB, Filchak K, Niedbalski J, Romero-Severson J (2003) Evidence for inversion polymorphism related to sympatric host race formation in the apple maggot fly, Rhagoletis pomonella. Genetics 163(3):939–953
Follett PA, McQuate GT (2001) Accelerated development of quarantine treatments for insects on poor hosts. J Econ Entomol 94:1005–1011
Franz G (2005) Genetic sexing strains in Mediterranean fruit fly, an example for other species amenable to large-scale rearing for the sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS (eds) Sterile insect technique: principles and practice in area-wide integrated pest management. Springer, Dordrecht, The Netherlands, pp 427–452
Garcia-Martinez V, Hernandez-Ortiz E, Zepeta-Cisneros CS, Robinson AS, Zacharopoulou A, Franz G (2009) Mitotic and polytene analysis in the Mexican fruit fly, Anastrepha ludens (Loew) (Diptera: Tephritidae). Genome 52:1–11
Gariou-Papalexiou A, Gourzi P, Delprat A, Kritikou D, Rapti K, Chrysanthakopoulou B, Mintzas A, Zacharopoulou A (2002) Polytene chromosomes as tools in the genetic analysis of the Mediterranean fruit fly, Ceratitis capitata. Genetica 116:59–71
Guelbeogo WM, Grushko O, Boccolini D, Ouédraogo PA, Besansky NJ, Sagnon NF, Costantini C (2005) Chromosomal evidence of incipient speciation in the Afrotropical malaria mosquito Anopheles funestus. Med Vet Entomol 19:46–458
Handler AM (2003) Isolation and analysis of a new hopper hAT transposon from the Bactrocera dorsalis white eye strain. Genetica 118:17–24
Handler AM, Gomez SP (1997) A new hobo, Ac, Tam3 transposable element, hopper, from Bactrocera dorsalis is distantly related to hobo and Ac. Gene 185:133–135
Hoffman AA, Rieseberg LH (2008) Revisiting the impact of inversions in evolution: from population genetic markers to drivers of adaptive shifts and speciation? Annu Rev Ecol Evol Syst 39:21–42
Holt RA, Subramanian GM, Halpern A, Sutton GG et al (2002) The genome sequence of the malaria mosquito Anopheles gambiae. Science 298:129–149
Hunwattanakul N, Baimai V (1994) Mitotic karyotypes of four species of fruit flies (Bactrocera) in Thailand. Kasetsart J (Nat Sci) 28:142–148
Jamnongluk W, Kittayapong P, Baimai V, O’Neill S (2002) Wolbachia infections of Tephritid fruit flies: molecular evidence for five distinct strains in a single host species. Curr Microb 45:255–260
Jamnongluk W, Baimai V, Kittayapong P (2003) Molecular evolution of tephritid fruit flies in the genus Bactrocera based on the cytochrome oxidase I gene. Genetica 119:19–25
Kounatidis I, Papadopoulos N, Bourtzis K, Mavragani-Tsipidou P (2008) Genetic and cytogenetic analysis of the fruit fly Rhagoletis cerasi (Diptera: Tephritidae). Genome 51:479–491
Krimbas CB, Powell JR (1992) Drosophila inversion polymorphism. CRC Press, Boca Raton, FL., USA
Kulathinal RJ, Stevison LS, Noor MAF (2008) The genomics of speciation in Drosophila: diversity, divergence, and introgression estimated using low-coverage genome sequencing. PLoS Genet 5(7):e1000550
Lacovaara S, Saura A (1982) Evolution and speciation in the Drosophila obscura group. In: Ashburner M, Carson HL, Thompson JN Jr (eds) The genetics and biology of Drosophila. Academic Press, London, 3b, pp 1–59
Lawson AE, McQuire DJ, Yeates DH, Drew RAI, Clarke AR (2003) Dorsalis: an interactive identification tool to fruit flies of the Bactrocera dorsalis complex. CDROM publication, Griffith Univ. Brisbane, Aust
Lefevre G (1976) A photographic representation and interpretation of the polytene chromosomes of Drosophila melanogaster salivary glands. In: Ashburner M, Novitski E (eds) The genetics and biology of Drosophila. Academic Press, London, 1a, pp 31–66
Lemeunier F, David JR, Tsakas L, Ashburner M (1986) The melanogaster species group. In: Ashburner M, Carson HL, Thopson JN (eds) The genetics and biology of Drosophila. Academic Press, London, 1e, pp 147–256
Lyttle TW, Haymer DS (1992) The role of the transposable element hobo in the origin of endemic inversions in wild populations of Drosophila melanogaster. Genetica 86:113–126
Machado CA, Kliman RM, Markert JA, Hey J (2002) Inferring the history of speciation from multilocus DNA sequence data: the case of Drosophila pseudoobscura and close relatives. Mol Biol Evol 19:472–488
Machado LPB, Madi-Ravazzi L, Tadei WJ (2006) Reproductive relationships and degree of synapsis in the polytene chromosomes of the Drosophila buzzatti species cluster. Braz J Biol 66:279–293
Machado CA, Matzkin LM, Reed LK, Markow TA (2007) Multilocus nuclear sequences reveal intra- and interspecific relationships among chromosomally polymorphic species of cactophilic Drosophila. Mol Ecol 16:3009–3024
Malavasi A, van Sauers-Muller A, Midgarden D, Kellman V, Didelot D, Caplong Ph, Ribeiro O (2000) Regional programme for the eradication of the Carambola fruit fly in South America. In: Tan KH (ed) Area-wide control of fruit flies and other insect pests. Penerbit Universiti Sains Malaysia, Penang, pp 395–399
Mathiopoulos KD, della Torre A, Santolamazza F, Predazzi V, Petrarca V, Coluzzi M (1999) Are chromosomal inversions induced by transposable elements? A paradigm from the malaria mosquito Anopheles gambiae. Parassitol 41:106–129
Mau MFA (2007) Bactrocera dorsalis (Hendel). Crop knowledge master. http://www.extento.hawaii.edu/Kbase/Crop/Type/bactro_d.htm
Mavragani-Tsipidou P, Karamanlidou G, Zacharopoulou A, Koliais S, Kastritsis C (1992) Mitotic and polytene chromosome analysis in Dacus oleae (Diptera: Tephritidae). Genome 35:373–378
McCombs SD, Saul SH (1992) Linkage analysis of genetic markers in the oriental fruit fly. In: McPheron BA, Steck GJ (eds) Fruit fly pests: a world assessment and management. St. Lucie Press, Fl, pp 231–235
McCombs SD, Saul SH (1995) Translocation based genetic sexing system for the oriental fruit fly (Diptera-Tephritidae) based on pupal color dimorphism. Ann Entomol Soc Am 88:695–698
McInnis DO, Rendon P, Jang E, van Sauers-Muller A, Sugayama R, Malavasi A (1999) Interspecific mating of introduced, sterile Bactrocera dorsalis with wild B. carambolae (Diptera: Tephritidae) in Suriname: a potential case for cross-species sterile insect technique. Ann Entomol Soc Am 92:758–765
Muller HJ (1940) Bearings of the Drosophila work on systematics. In: Huxley JS (ed) The new systematics. Oxford University Press (Clarendon), London and New York, pp 185–268
Muraji M, Nakahara S (2001) Phylogenetic relationships among fruit flies, Bactrocera (Diptera, Tephritidae), based on mitochondrial rDNA sequences. Insect Mol Biol 10:549–559
Muraji M, Nakahara S (2002) Discrimination among pest species of Bactrocera (Diptera: Tephritidae) based on PCR-RFLP of the mitochondrial DNA. Appl Entomol Zool 37:437–446
Naeole CKM, Haymer DS (2003) Use of oligonucleotide arrays for molecular taxonomic studies of closely related species in the oriental fruit fly complex. Mol Ecol Notes 3:662–665
Nakahara S, Tsuchiya T, Sato M, Masaki M, Kaneda M (2000) Research on infestation to many kinds of plants by the pests of quarantine importance. Bactrocera dorsalis complex. Res Bull Plant Prot Serv Jpn 36:53–56
Nakahara S, Kato H, Kaneda M, Sugimoto T, Muraji M (2001) Identification of Bactrocera dorsalis complex species (Diptera: Tephritidae) by PCR-RFLP analysis. II. A study of genetic variation in B. dorsalis complex (Philippines population) and B. dorsalis (Taiwan population). Res Bull Plant Prot Serv Jpn 37:69–73
Nakahara S, Kobashigawa Y, Muraji M (2008) Genetic variation among and within populations of the Oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae), detected by PCR-RFLP of the mitochondrion control region. Appl Entomol Zool 43:457–465
Navarro A, Barton NH (2003) Chromosomal speciation and molecular divergence—accelerated evolution in rearranged chromosomes. Science 300:321–324
Noor MAF, Gratos KL, Bertucci LA, Reiland J (2001) Chromosomal inversions and the reproductive isolation of species. PNAS 98:12084–12088
Noor MAF, Garfield DA, Schaeffer SW, Machado CA (2007) Divergence between the Drosophila pseudoobscura and D. persimilis genome sequences in relation to chromosomal inversions. Genetics 177:1417–1428
Oliver KR, Greene WK (2009) Transposable elements: powerful facilitators of evolution. Bioess 31:703–714
Ranz JM, Maurin D, Chan YS, Von Grotthuss M, Hillier LW, Roote J, Ashburner M, Bergman CM (2007) Principles of genome evolution in the Drosophila melanogaster species group. PLoS Biol 5e152:1369–1381
Richards S, Liu Y, Bettencourt BR, Hradecky P et al (2005) Comparative genome sequencing of Drosophila pseudoobscura: chromosomal, gene, and cis-element evolution. Genome Res 15:1–18
Robinson AS, Franz G, Fisher K (1999) Genetic sexing strains in the medfly, Ceratitis capitata: development, mass rearing and field application. Trends Entomol 2:81–104
Runcie DE, Noor MAF (2009) Sequence signatures of a recent chromosomal rearrangement in Drosophila mojavensis. Genetica 136:5–11
Seewootuthum SI, Permaloo S, Gungah B, Soonnoo AR, Alleck M (2000) Eradication of an exotic fruit fly from Mauritius. In: Tan KH (ed) Area-wide control of fruit flies and other insect pests. Penerbit Universiti Sains Malaysia, Penang, pp 389–394
Selivon D, Perondini ALP (1997) Evaluation of techniques for C and ASG banding of the mitotic chromosomes of Anastrepha species (Diptera: Tephritidae). Braz J Genet 20:651–653
Tan KH (2000) Behaviour and chemical ecology of Bactrocera flies. In: Tan KH (ed) Area-wide control of fruit flies and other insect pests. Penerbit Universiti Sains Malaysia, Pulau, Penang, pp 647–656
Wee SL, Tan KH (2005) Evidence of natural hybridization between two sympatric sibling species of Bactrocera dorsalis complex based on pheromone analysis. J Chem Ecol 31:845–858
White IM, Elson-Harris M (1992) Fruit flies of economic significance: their identification and bionomics. CAB International, Oxford, UK
Yu DJ, Xu L, Nardi F, Li JG, Zhang RJ (2007) The complete nucleotide sequence of the mitochondrial genome of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Gene 396:66–74
Zacharopoulou A (1990) Polytene chromosome maps in the medfly Ceratitis capitata. Genome 33:184–197
Zhao JT, Fommer M, Sved J, Zacharopoulou A (1998) Mitotic and polytene chromosome analyses in the Queensland fruit fly, Bactrocera tryoni (Diptera: Tephritidae). Genome 41:510–526
Zhimulev IF, Belyaeva ES, Semeshin VF, Koryakov DE, Demakov SA, Demakova OV, Pokholkova GV, Andreyeva EN (2004) Polytene chromosomes: 70 years of genetic research. Int Rev Cytol 241:203–275
Acknowledgments
This work forms part of the Joint FAO/IAEA research programme for the development of improved control methodologies against fruit fly pest species. We would also like to thank the two anonymous reviewers for their significant comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zacharopoulou, A., Augustinos, A.A., Sayed, W.A.A. et al. Mitotic and polytene chromosomes analysis of the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Genetica 139, 79–90 (2011). https://doi.org/10.1007/s10709-010-9495-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-010-9495-3