Abstract
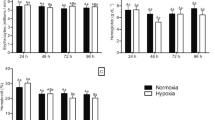
Hypoxia is one of the most significant threats to biodiversity in aquatic systems. The ability of high-latitude fish to tolerate hypoxia with histological and physiological responses is mostly unknown. We address this knowledge gap by investigating the effects of exposures to different oxygen levels using Phoxinus lagowskii (a high-latitude, cold-water fish) as a model. Fish were exposed to different oxygen levels (0.5 mg/L and 3 mg/L) for 24 h. The loss of equilibrium (LOE), an indicator of acute hypoxia tolerance, was 0.21 ± 0.01 mg/L, revealing the ability of fish to tolerate low-oxygen conditions. We sought to determine if, in P. lagowskii, the histology of gills and liver, blood indicators, enzyme activities of carbohydrate and lipid metabolism, and antioxidants changed to relieve stress in response to acute hypoxia. Notably, changes in vigorous jumping behavior under low oxygen revealed the exceptional hypoxia acclimation response compared with other low-latitude fish. A decrease in blood parameters, including RBC, WBC, and Hb, as well as an increase in MCV was observed compared to the controls. The increased total area in lamella and decreased ILCM volume in P. lagowskii gills were detected in the present study. Our results also showed the size of vacuoles in the livers of the hypoxic fish shrunk. Interestingly, an increase in the enzyme activity of lipid metabolism but not glucose metabolism was observed in the groups exposed to hypoxia at 6 h and 24 h. After combining histology and physiology results, our findings provide evidence that lipid metabolism plays a crucial role in enhancing hypoxia acclimation in P. lagowskii. Additionally, SOD activity significantly increased during hypoxia, suggesting the presence of an antioxidant response of P. lagowskii during hypoxia. High expression levels of lipogenesis and lipolysis-related genes were detected in the 6 h 3 mg/L and 24 h 3 mg/L hypoxia group. Enhanced expression of lipid-metabolism genes (ALS4, PGC-1, and FASN) was detected during hypoxia exposure. Together, these data suggest that P. lagowskii’s ability to tolerate hypoxic events is likely mediated by a comprehensive strategy.








Similar content being viewed by others
References
Abdel-Tawwab M, Monier MN, Hoseinifar SH, Faggio C (2019) Fish response to hypoxia stress: growth, physiological, and immunological biomarkers. Fish Physiol Biochem 45:997–1013
Andreeva A, Skverchinskaya E, Gambaryan S, Soldatov A, Mindukshev I (2018) Hypoxia inhibits the regulatory volume decrease in red blood cells of common frog (Rana temporaria). Comp Biochem Physiol A Mol Integr Physiol 219–220:44–47
Augustus AS, Kako Y, Yagyu H, Goldberg IJ (2003) Routes of FA delivery to cardiac muscle: modulation of lipoprotein lipolysis alters uptake of TG-derived FA. Am J Physiol Endocrinol Metab 284:E331–E339
Benson BB, Krause D Jr (1984) The concentration and isotopic fractionation of oxygen dissolved in freshwater and seawater in equilibrium with the atmosphere1. Limnol Oceanogr 29:620–632
Borowiec BG, Darcy KL, Gillette DM, Scott GR (2015) Distinct physiological strategies are used to cope with constant hypoxia and intermittent hypoxia in killifish (Fundulus heteroclitus). J Exp Biol 218:1198–1211
Boyd C, Tucker C (1998) Pond Aquaculture water quality management. Kluwer Academic Publishers, Norwell, pp 87–153
Boyko M, Melamed I, Gruenbaum BF, Gruenbaum SE, Ohayon S, Leibowitz A, Brotfain E, Shapira Y, Zlotnik A (2012) The effect of blood glutamate scavengers oxaloacetate and pyruvate on neurological outcome in a rat model of subarachnoid hemorrhage. Neurotherapeutics 9:649–657
Brauner C, Ballantyne C, Randall D, Val A (2011) Air breathing in the armoured catfish (Hoplosternum littorale) as an adaptation to hypoxic, acidic, and hydrogen sulphide rich waters. Can J Zool 73:739–744
Campbell L, Rice J (2014) Effects of hypoxia-induced habitat compression on growth of juvenile fish in the Neuse River Estuary, North Carolina, USA. Mar Ecol Prog Ser 497:119–213
Cao Y, Murphy K, McIntyre T, Zimmerman G, Prescott S (2000) Expression of fatty acid-CoA ligase 4 during development and in brain. FEBS Lett 467:263–267
Chapman LJ, McKenzie DJ (2009) Chapter 2 behavioral responses and ecological consequences. In: Richards JG, Farrell AP, Brauner CJ (eds) Fish Physiology 27:25–77
Chirala S, Chang H, Matzuk M, Abu-Elheiga L, Mao J, Mahon K, Finegold M, Wakil S (2003) Fatty acid synthesis is essential in embryonic development: fatty acid synthase null mutants and most of the heterozygotes die in utero. Proc Natl Acad Sci U S A 100:6358–6363
Collins GM, Clark TD, Carton AG (2016) Physiological plasticity v. inter-population variability: understanding drivers of hypoxia tolerance in a tropical estuarine fish. Mar Freshw Res 67:1575–1582
Connett RJ, Honig CR, Gayeski TE, Brooks GA (1990) Defining hypoxia: a systems view of VO2, glycolysis, energetics, and intracellular PO2. J Appl Physiol 68:833–842
Cooper RU, Clough LM, Farwell MA, West TL (2002) Hypoxia-induced metabolic and antioxidant enzymatic activities in the estuarine fish Leiostomus xanthurus. J Exp Mar Biol Ecol 279:1–20
Cossins A, Gibson JS (1997) Volume -sensitive transport systems and Volume homeostasis in vertebrate red blood cells. J Exp Biol 200:343–352
Dan XM, Yan GJ, Zhang AJ, Cao ZD, Fu SJ (2014) Effects of stable and diel-cycling hypoxia on hypoxia tolerance, postprandial metabolic response, and growth performance in juvenile qingbo (Spinibarbus sinensis). Aquaculture 428-429:21–28
Dawson NJ, Storey KB (2012) An enzymatic bridge between carbohydrate and amino acid metabolism: regulation of glutamate dehydrogenase by reversible phosphorylation in a severe hypoxia-tolerant crayfish. J Comp Physiol B 182:331–340
Dhillon RS, Yao L, Matey V, Chen BJ, Zhang AJ, Cao ZD, Fu SJ, Brauner CJ, Wang YS, Richards JG (2013) Interspecific differences in hypoxia-induced gill remodeling in carp. Physiol Biochem Zool 86:727–739
Diaz R, Breitburg D (2009) Chapter 1 The hypoxic environment. Fish Physiol 27:1–23
Díaz A, González-Castro M, García A, Diaz de Astarloa J, Figueroa D (2008) Gross morphology and surface ultrastructure of the gills of Odontesthes argentinensis (Actinopterygii, Atherinopsidae) from a Southwestern Atlantic coastal lagoon. Tissue Cell 41:193–198
Diaz M, Vraskou Y, Gutierrez J, Planas JV (2009) Expression of rainbow trout glucose transporters GLUT1 and GLUT4 during in vitro muscle cell differentiation and regulation by insulin and IGF-I. Am J Phys Regul Integr Comp Phys 296:R794–R800
Ding J, Liu C, Luo S, Zhang Y, Gao X, Wu X, Shen W, Zhu J (2019) Transcriptome and physiology analysis identify key metabolic changes in the liver of the large yellow croaker (Larimichthys crocea) in response to acute hypoxia. Ecotox Environ Safe 189:109957
Dolci GS, Rosa HZ, Vey LT, Pase CS, Barcelos RCS, Dias VT, Loebens L, Dalla Vecchia P, Bizzi CA, Baldisserotto B, Burger ME (2017) Could hypoxia acclimation cause morphological changes and protect against Mn-induced oxidative injuries in silver catfish (Rhamdia quelen) even after reoxygenation? Environ Pollut 224:466–475
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trümbach D, Mao G, Qu F, Bayir H, Füllekrug J, Scheel CH, Wurst W, Schick JA, Kagan VE, Angeli JPF, Conrad M (2016) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13:91–98
Evans D, Piermarini P, Choe K (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177
Ficke AD, Myrick CA, Hansen LJ (2007) Potential impacts of global climate change on freshwater fisheries. Rev Fish Biol Fish 17:581–613
Fritsche R, Nilsson S (1993) Cardiovascular and ventilatory control during hypoxia. Fish Ecophysiology 9:180–206
Fu SJ, Fu C, Yan GJ, Cao ZD, Zhang AJ, Pang X (2014) Interspecific variation in hypoxia tolerance, swimming performance and plasticity in cyprinids that prefer different habitats. J Exp Biol 217:590–597
Garduño Paz MV, Méndez Sánchez JF, Burggren W, García Martínez JLA (2020) Metabolic rate and hypoxia tolerance in Girardinichthys multiradiatus (Pisces: Goodeidae), an endemic fish at high altitude in tropical Mexico. Comp Biochem Physiol A Mol Integr Physiol 239:110576
Gonzalez R, McDonald D (1994) The relationship between oxygen uptake and ion loss in fish from diverse habitats. J Exp Biol 190:95–108
Gorr T, Gerst D, Hu J, Hermes-Lima M, Welker A, Terwilliger N, Wren J, Viney M, Morris S, Nilsson G, Deten A, Soliz J, Gassmann M (2010) Hypoxia tolerance in animals: biology and application. Physiol Biochem Zool 83:733–752
Gottlieb M, Wang Y, Teichberg V (2003) Blood-mediated scavenging of cerebrospinal fluid glutamate. J Neurochem 87:119–126
Gracey A, Troll J, Somero G (2001) Hypoxia-induced expression profiling in the euryoxic fish Gillichthys mirabilis. Proc Natl Acad Sci U S A 98:1993–1998
Gracey A, Lee T-H, Higashi R, Fan T (2011) Hypoxia-induced mobilization of stored triglycerides in the euryoxic goby Gillichthys mirabilis. J Exp Biol 214:3005–3012
Guo W, Zhang Y, Tong G, Chen Z, Qi P, Yin J (2015) Embryonic development of wild Phoxinus lagowskii Dybowskii collected from Suifen River in Heilong River Valley (Chinese with English abstract). Chin J Ecol 34:2530–2536
Hägerhäll C (1997) Succinate: quinone oxidoreductases -variations on a conserved theme. Biochim Biophys Acta 1320:107–141
He W, Cao Z-D, Fu S-J (2014) Effect of temperature on hypoxia tolerance and its underlying biochemical mechanism in two juvenile cyprinids exhibiting distinct hypoxia sensitivities. Comp Biochem Physiol A Mol Integr Physiol 187:232–241
Heinrichs-Caldas W, Campos D, Nazaré P-S, Almeida-Val V (2018) Oxygen-dependent distinct expression of HIF-1α gene in aerobic and anaerobic tissues of the Amazon Oscar, Astronotus crassipinnis. Comp Biochem Physiol B: Biochem Mol Biol 227:31–38
Herbert NA, Steffensen JF (2005) The response of Atlantic cod, Gadus morhua, to progressive hypoxia: fish swimming speed and physiological stress. Mar Biol 147:1403–1412
Heshmati J, Golab F, Morvaridzadeh M, Potter E, Akbari-Fakhrabadi M, Farsi F, Tanbakooei S, Shidfar F (2020) The effects of curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor γ coactivator 1α gene expression in polycystic ovarian syndrome (PCOS) patients: a randomized placebo-controlled clinical trial. Diabetes Metab Syndr Clin Res Rev 14:77–82
Hou ZS, Wen HS, Li JF, He F, Li Y, Qi X (2020) Environmental hypoxia causes growth retardation, osteoclast differentiation and calcium dyshomeostasis in juvenile rainbow trout (Oncorhynchus mykiss). Sci Total Environ 705:135272
Huntingford F, Adams C (2005) Behavioural syndromes in farmed fish: Implications for production and welfare. Behaviour 142:1207–1221
Jimenez A, Braun E, Tobin K (2019) How does chronic temperature exposure affect hypoxia tolerance in sheepshead minnows’ (Cyprinodon variegatus variegatus) ability to tolerate oxidative stress? Fish Physiol Biochem 45:499–510
Jin J, Yang Y, Zhu X, Han D, Liu H, Xie S (2018) Effects of glucose administration on glucose and lipid metabolism in two strains of gibel carp (Carassius gibelio). Gen Comp Endocrinol 267:18–28
Johannsson OE, Giacomin M, Sadauskas-Henrique H, Campos DF, Braz-Mota S, Heinrichs-Caldas WD, Baptista R, Wood CM, Almeida-Val VMF, Val AL (2018) Does hypoxia or different rates of re-oxygenation after hypoxia induce an oxidative stress response in Cyphocharax abramoides (Kner 1858), a Characid fish of the Rio Negro? Comp Biochem Physiol A Mol Integr Physiol 224:53–67
Johnston I, Bernard L (1982) Ultrastructure and metabolism of skeletal muscle fibres in the tench: effects of long-term acclimation to hypoxia. Cell Tissue Res 227:179–199
Kwasek K, Rimoldi S, Cattaneo A, Parker T, Dabrowski K, Terova G (2017) The expression of hypoxia-inducible factor-1α gene is not affected by low oxygen conditions in yellow perch (Perca flavescens) juveniles. Fish Physiol Biochem 43:849–862
Labboun S, Tercé-Laforgue T, Roscher A, Bedu M, Restivo F, Velanis C, Skopelitis D, Moschou P, Moshou P, Roubelakis-Angelakis K, Suzuki A, Hirel B (2009) Resolving the role of plant glutamate dehydrogenase. I. in vivo real time nuclear magnetic resonance spectroscopy experiments. Plant Cell Physiol 50:1761–1773
Laursen DC, Olsén HL, Ruiz-Gomez ML, Winberg S, Höglund E (2011) Behavioural responses to hypoxia provide a non-invasive method for distinguishing between stress coping styles in fish. Appl Anim Behav Sci 132:211–216
Le P, Do H, Nyengaard J, Bayley M (2016) Gill remodelling and growth rate of striped catfish Pangasianodon hypophthalmus under impacts of hypoxia and temperature. Comp Biochem Physiol A Mol Integr Physiol 203:288–296
Leveelahti L, Rytkönen KT, Renshaw GMC, Nikinmaa M (2014) Revisiting redox-active antioxidant defenses in response to hypoxic challenge in both hypoxia-tolerant and hypoxia-sensitive fish species. Fish Physiol Biochem 40:183–191
Li M, Wang X, Qi C, Li E, Du Z, Qin JG, Chen L (2018) Metabolic response of Nile tilapia (Oreochromis niloticus) to acute and chronic hypoxia stress. Aquaculture 495:187–195
Ma N, Nicholson C, Wong M, Holloway A, Hardy D (2013) Fetal and neonatal exposure to nicotine leads to augmented hepatic and circulating triglycerides in adult male offspring due to increased expression of fatty acid synthase. Toxicol Appl Pharm 275:1–11
Ma XY, Qiang J, He J, Gabriel NN, Xu P (2015) Changes in the physiological parameters, fatty acid metabolism, and SCD activity and expression in juvenile GIFT tilapia (Oreochromis niloticus) reared at three different temperatures. Fish Physiol Biochem 41:937–950
Mahfouz ME, Hegazi MM, El-Magd MA, Kasem EA (2015) Metabolic and molecular responses in Nile tilapia, Oreochromis niloticus during short and prolonged hypoxia. Mar Freshw Behav Physiol 48:319–340
Mandic M, Speers-Roesch B, Richards J (2013) Hypoxia tolerance in sculpins is associated with high anaerobic enzyme activity in brain but not in liver or muscle. Physiol Biochem Zool 86:92–105
Mann LI (1970) Effects in sheep of hypoxia on levels of lactate, pyruvate, and glucose in blood of mothers and fetus. Pediatr Res 4:46–54
Mariana L, Fabrizius A, Czech-Damal N, Folkow L, Burmester T (2017) Transcriptome analysis identifies key metabolic changes in the hooded seal (Cystophora cristata) brain in response to hypoxia and reoxygenation. PLoS One 12:e0169366
Martinez A-S, Cutler CP, Wilson GD, Phillips C, Hazon N, Cramb G (2005) Regulation of expression of two aquaporin homologs in the intestine of the European eel: effects of seawater acclimation and cortisol treatment. Am J Phys Regul Integr Comp Phys 288:R1733–R1743
Mashek D, Li L, Coleman R (2007) Long-chain acyl-CoA synthetases and fatty acid channeling. Futur Lipidol 2:465–476
Matey V, Richards JG, Wang Y, Wood CM, Rogers J, Davies R, Murray BW, Chen XQ, Du J, Brauner CJ (2008) The effect of hypoxia on gill morphology and ionoregulatory status in the Lake Qinghai scaleless carp, Gymnocypris przewalskii. J Exp Biol 211:1063–1074
Morley SA, Peck Ls Fau-Miller AJ, Miller Aj Fau-Pörtner HO, Pörtner HO (2007) Hypoxia tolerance associated with activity reduction is a key adaptation for Laternula elliptica seasonal energetics. Oecologia 153:29–36
Mustafa S, Davies S, Jha A (2012) Determination of hypoxia and dietary copper mediated sub-lethal toxicity in carp, Cyprinus carpio, at different levels of biological organisation. Chemosphere 87:413–422
Nierenberg AA, Ghaznavi SA, Sande Mathias I, Ellard KK, Janos JA, Sylvia LG (2018) Peroxisome proliferator-activated receptor gamma coactivator-1 alpha as a novel target for bipolar disorder and other neuropsychiatric disorders. Biol Psychiatry 83:761–769
Nilsson S (1986) Control of gill blood flow. In: Nilsson S, Holmgren S (eds) Fish physiology: recent advances. Croom Helm, London, pp 86–101
Nilsson GE, Dymowska A, Stecyk JAW (2012) New insights into the plasticity of gill structure. Respir Physiol Neurobiol 184:214–222
Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S (2001) Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J Physiol 532:3–16
Polakof S, Panserat S, Soengas JL, Moon TW (2012) Glucose metabolism in fish: a review. J Comp Physiol B 182:1015–1045
Pothoven S, Vanderploeg H, Ludsin S, Höök T, Brandt S (2009) Feeding ecology of emerald shiners and rainbow smelt in central Lake Erie. J Great Lakes Res 35:190–198
Randall DJ, Daxboeck C (1984) Oxygen and carbon dioxide transfer across fish gills. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic Press, Orlando, pp 263–314
Richards J (2011) Physiological, behavioral and biochemical adaptations of intertidal fishes to hypoxia. J Exp Biol 214:191–199
Rimoldi S, Terova G, Ceccuzzi P, Marelli S, Antonini M, Saroglia M (2012) HIF-1α mRNA levels in Eurasian perch (Perca fluviatilis) exposed to acute and chronic hypoxia. Mol Biol Rep 39:4009–4015
Roberts JJ (2010) The ecological consequences of hypoxia for yellow perch (Perca flavescens) in Lake Erie. PhD Dissertation. University of Michigan, Ann Arbor
Roberts JJ, Grecay PA, Ludsin SA, Pothoven SA, Vanderploeg HA, Höök TO (2012) Evidence of hypoxic foraging forays by yellow perch (Perca flavescens) and potential consequences for prey consumption. Freshw Biol 57:922–937
Rocha-Santos C, Bastos FF, Dantas RF, Hauser-Davis RA, Rodrigues LC, Cunha Bastos VLF, Cunha Bastos J (2018) Glutathione peroxidase and glutathione S-transferase in blood and liver from a hypoxia-tolerant fish under oxygen deprivation. Ecotoxicol Environ Saf 163:604–611
Rye P, Lamarr W (2015) Measurement of glycolysis reactants by high-throughput solid phase extraction with tandem mass spectrometry: characterization of pyrophosphate-dependent phosphofructokinase as a case study. Anal Biochem 482:40–47
Saroglia M, Cecchini S, Terova G, Caputo AR, De Stradis A (2000) Influence of environmental temperature and water oxygen concentration on gas diffusion distance in sea bass (Dicentrarchus labrax, L.). Fish Physiol Biochem 23:55–58
Saroglia M, Terova G, De Stradis A, Caputo AR (2002) Morphometric adaptations of sea bass gills to different dissolved oxygen partial pressure. J Fish Biol 60:1423–1420
Scott AL, Rogers WA (2006) Haematological effects of prolonged hypoxia on channel catfish Ictalurus punctatus (Rafinesque). J Fish Biol 18:591–601
Scott-Taggart C, Van Cauwenberghe O, D M, Shelp B (2002) Regulation of Γ-aminobutyric acid synthesis in situ by glutamate availability. Physiol Plant 106:363–369
Serebrovska TV, Portnychenko AG, Portnichenko VI, Xi L, Egorov E, Antoniuk-Shcheglova I, Naskalova S, Shatylo VB (2019) Effects of intermittent hypoxia training on leukocyte pyruvate dehydrogenase kinase 1 (PDK-1) mRNA expression and blood insulin level in prediabetes patients. Eur J Appl Physiol 119:813–823
Shi Z, Liu K, Zhang S, Xu H, Liu H (2019) Spatial distributions of mesozooplankton biomass, community composition and grazing impact in association with hypoxia in the Pearl River Estuary. Estuar Coast Shelf Sci 225:106237
Silva GS, Matos LV, Freitas JOS, Campos DF, Almeida e Val VMF (2019) Gene expression, genotoxicity, and physiological responses in an Amazonian fish, Colossoma macropomum (CUVIER 1818), exposed to Roundup® and subsequent acute hypoxia. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 222:49–58
Sollid J, De Angelis P, Gundersen K, Nilsson GE (2003) Hypoxia induces adaptive and reversible gross morphological changes in crucian carp gills. J Exp Biol 206:3667–3673
Song W, Zhong C, Yuan Y, Zhu Q, Wang Y, Yin H, Li D, Zhang Z, Shu G, Yang C, Du H, Jiang X, Zhao X (2020) Peroxisome proliferator-activated receptor-coactivator 1-beta (PGC-1β) modulates the expression of genes involved in adipogenesis during preadipocyte differentiation in chicken. Gene 741:144516
Speers-Roesch B, Sandblom E, Lau G, Farrell A, Richards J (2009) Effects of environmental hypoxia on cardiac energy metabolism and performance in tilapia. American journal of physiology. Am J Physiol Regul Integr Comp Physiol 298:R104–R119
Sun Q, Wang D, Wei Q (2014) The complete mitochondrial gemone of Phoxinus lagowskii (Teleostei, Cypriniformes: Cyprinidae). Mitochondrial DNA 27:830–831
Sun JL, Zhao LL, Wu H, Liu Q, Liao L, Luo J, Lian WQ, Cui C, Jin L, Ma JD, Li MZ, Yang S (2020) Acute hypoxia changes the mode of glucose and lipid utilization in the liver of the largemouth bass (Micropterus salmoides). Sci Total Environ 713:135157
Terova G, Rimoldi S, Corà S, Bernardini G, Gornati R, Saroglia M (2008) Acute and chronic hypoxia affects HIF-1α mRNA levels in sea bass (Dicentrarchus labrax). Aquaculture 279:150–159
Thomé RG, de Oliveira Cardoso IC, de Oliveira SE, Santos HBD (2018) Oogenesis is accompanied by cyclic morphological changes in hepatocytes of Neotropical freshwater fish Piabina argentea. Anat Histol Embryol 47:466–474
Tzaneva V, Gilmour KM, Perry SF (2011) Respiratory responses to hypoxia or hypercapnia in goldfish (Carassius auratus) experiencing gill remodelling. Respir Physiol Neurobiol 175:112–120
Val AL, Gomes KRM, de Almeida-Val VMF (2015) Rapid regulation of blood parameters under acute hypoxia in the Amazonian fish Prochilodus nigricans. Comp Biochem Physiol A Mol Integr Physiol 184:125–131
Van Hellemond JJ, Tielens AGM (1994) Expression and functional properties of fumarate reductase. Biochem J 304:321–331
Vaquer-Sunyer R, Duarte CM (2008) Thresholds of hypoxia for marine biodiversity. Proc Natl Acad Sci 105:15452–15457
Wang QF, Shen WL, Hou CC, Liu C, Wu XF, Zhu JQ (2017a) Physiological responses and changes in gene expression in the large yellow croaker Larimichthys crocea following exposure to hypoxia. Chemosphere 169:418–427
Wang X, Liu S, Dunham R, Liu Z (2017b) Effects of strain and body weight on low-oxygen tolerance of channel catfish (Ictalurus punctatus). Aquac Int 25:1645–1652
Wang Q, Luo S, Ghonimy A, Chen Y, Guo Z, Liu H, Zhang D (2019) Effect of dietary l-carnitine on growth performance and antioxidant response in Amur minnow (Phoxinus lagowskii Dybowskii). Aquac Nutr 25:749–760
Wannamaker C, Rice J (2000) Effects of hypoxia on movements and behaviour of selected estuarine organisms from the Southeastern United States. J Exp Mar Biol Ecol 249:145–163
Westerterp M, Haan W, Berbée J, Havekes L, Rensen P (2006) Endogenous apoC-I increases hyperlipidemia in apoE-knockout mice by stimulating VLDL production and inhibiting LPL. J Lipid Res 47:1203–1211
Wu CB, Liu ZY, Li FG, Chen J, Jiang XY, Zou SM (2017) Gill remodeling in response to hypoxia and temperature occurs in the hypoxia sensitive blunt snout bream (Megalobrama amblycephala). Aquaculture 479:479–486
Wu CB, Zheng GD, Zhao XY, Zhou S, Zou SM (2020) Hypoxia tolerance in a selectively bred F4 population of blunt snout bream (Megalobrama amblycephala) under hypoxic stress. Aquaculture 518:734484
Yang H, Cao ZD, Fu SJ (2013) The effects of diel-cycling hypoxia acclimation on the hypoxia tolerance, swimming capacity and growth performance of southern catfish (Silurus meridionalis). Comp Biochem Physiol A Mol Integr Physiol 165:131–138
Zhao LL, Sun JL, Liang J, Liu Q, Luo J, Li ZQ, Yan TM, Zhou J, Yang S (2020) Enhancing lipid metabolism and inducing antioxidant and immune responses to adapt to acute hypoxic stress in Schizothorax prenanti. Aquaculture 519:734933
Availability of data and material
Not applicable.
Code availability
Not applicable.
Funding
The research was supported by the National Natural Science Foundation of China (31970496), Harbin Normal University Graduate Innovation Project (HSDSSCX2020-14), and Program of Natural Science of Heilongjiang Province of R. P. China (LH2019C040, C2018038).
Author information
Authors and Affiliations
Contributions
Yuting Yang: methodology, investigation, formal analysis, writing the original draft. Zhen Wang: methodology, investigation, formal analysis. Jing Wang: Methodology, formal analysis. Fengming Lyu: Methodology, Formal analysis. Kexin Xu: Methodology, Formal analysis. Weijie Mu: Conceptualization, Methodology, Investigation, Formal analysis, Writing original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
All experiments were performed in accordance with the NIH guidelines for the care and use of laboratory animals (NIH Publication No. 85-23 Rev. 1985) and were approved by the Ethics Committee of Harbin Normal University.
Consent to participate
All names in the author list have been involved in various stages of experimentation or writing.
Consent for publication
All of the authors agreed to submit the paper for publication in the Journal of Fish Physiology and Biochemistry.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 221 kb)
Rights and permissions
About this article
Cite this article
Yang, Y., Wang, Z., Wang, J. et al. Histopathological, hematological, and biochemical changes in high-latitude fish Phoxinus lagowskii exposed to hypoxia. Fish Physiol Biochem 47, 919–938 (2021). https://doi.org/10.1007/s10695-021-00947-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-021-00947-4