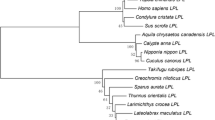
Abstract
A 4-week study was conducted to determine the effect of starvation on activities and mRNA expression of lipoprotein lipase (LPL) and hormone-sensitive lipase (HSL) in hybrid tilapia (Oreochromis niloticus × O. areus). The tissue samples were sampled once a week. Results showed that body weight (BW) and hepatosomatic index (HSI) were decreased significantly (P < 0.05) during starvation. The percentages of crude fat and crude protein in the whole body and the fat content in muscle decreased significantly (P < 0.05), while the rate of moisture and crude ash increased significantly (P < 0.05). The response of LPL, HSL activities and mRNA expression in tissues was tissue dependent. The activities of LPL and HSL in muscle at day 7 were elevated by 2.5 times (P < 0.05) and 11.8 times (P < 0.05) of the value at day 0, respectively, and both then decreased to pre-starvation levels at day 14 and finally stabilized at a certain level afterward. LPL and HSL mRNA abundance in muscle remained relatively stable between 0 and 14 day; then, a significant increase was seen after 14 days. In the liver, LPL activity maintained a significantly increasing trend during starvation, while HSL activity rose dramatically at day 7 of starvation by 2.35 times (P < 0.05) and finally stabilized at a certain level. The mRNA abundance of liver LPL increased significantly during the whole process of starvation (P < 0.05), whereas the mRNA abundance of liver HSL decreased significantly at day 7 of starvation, elevating significantly afterward (P < 0.05).





Similar content being viewed by others
References
Albalat A, Gómez-Requeni P, Rojas P, Médale F, Kaushik S, Vianen GJ, Van den Thillart G, Gutiérrez J, Pérez-Sánchez J, Navarro I (2005) Nutritional and hormonal control of lipolysis in isolated gilthead seabream (Sparus aurata) adipocytes. Am J Physiol Regul Integr Comp Physiol 289:R259–R265
Albalat A, Sánchez-Gurmaches J, Gutiérrez J, Navarro I (2006) Regulation of lipoprotein lipase activity in rainbow trout (Oncorhynchus mykiss) tissues. Gen Comp Endocrinol 146:226–235
Auwerx J, Leroy P, Schoonjans K (1992) Lipoprotein lipase: recent contributions from molecular biology. Crit Rev Clin Lab Sci 29(3):243–268
Bisbal GA, Bengtson DA (1995) Description of starving condition in summer flounder, Paralichthys dentatus, early history stage. Fish Bull 93:217–230
Black D, Love RM (1986) The sequential mobilisation and restoration of energy reserves in tissues of Atlantic cod during starvation and re-feeding. J Comp Physiol B Biochem Syst Environ Physiol 469–479
Black D, Kirkpatrick SA, Skinner ER (1983) Lipoprotein lipase and salt-resistant lipase activities in the livers of the rainbow trout and cod. Biochem Soc Trans 11:708–795
Bonnet M, Leroux C, Faulconnier Y (2000) Lipoprotein lipase activity and mRNA are up-regulated by refeeding in adipose tissue and cardiac muscle of sheep. J Nutr 130:749–756
Boone C, Mourot J, Gregoire F, Remacle C (2000) The adipose conversion process: regulation by extracellular and intracellular factors. Reprod Nutr Dev 40:325–358
Buckley LJ (1980) Changes in ribonucleic acid, deoxyribonucleic acid, and protein content during ontogenesis in winter flounder, Pseudopleuronectes americanus, and effect of starvation. Fish Bull 77:703–708
Burgaya F, Peinado J, Vilaro S (1989) Lipoprotein lipase activity in neonatal-rat liver cell types. Biochem J 259:159–166
Bustin SA (2000) Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 25(2):169–193
Doolittle MH, Ben-Zeev O, Elovson J (1990) The response of lipoprotein lipase to feeding and fasting. J Biol Chem 265:4570–4577
Einen O, Waagen B, Thomassen MS (1998) Starvation prior to slaughter in Atlantic salmon (Salmo salar): I. Effects on weight loss, body shape, slaughter and fillet-yield, proximate and fatty acid composition. Aquaculture 166:85–104
Enerback S, Semb H, Tavernier J, Bjursell G, Olivecrona T (1988) Tissue specific regulation of guinea-pig lipoprotein lipase; effect of nutritional state and tumor necrosis factor on mRNA levels in adipose tissue, heart and liver. Gene 64:97–106
Faulconnier Y, Bonne M, Bocquier F, Leroux C, Chilliard Y (2001) Effects of photoperiod and feeding level on adipose tissue and muscle lipoprotein lipase activity and mRNA level in dry non-pregnant sheep. Br J Nutr 85:299–306
Guderley H, Lapointe D, Bedard M, Dutil JD (2003) Metabolic priorities during starvation: enzyme sparing in liver and white muscle of Atlantic cod, Gadus morhua L. Comp Biochem Physiol Part A Mol Integr Physiol 135:347–356
Hansson O, Donsmark M, Ling C, Nevsten P, Danfelter M, Andersen JL, Galbo H, Holm C (2005) Transcriptome and proteome analysis of soleus muscle of hormone-sensitive lipase-null mice. J Lipid Res 46:2614–2623
Holm C, Belfrage P, Fredrikson G (1987) Immunological evidence for the presence of hormone-sensitive lipase in rat tissues other than adipose tissue. Biochem Biophys Res Commun 148:99–105
Holm C, Kirchgessner TG, Svenson KL, Fredrikson G, Nilsson S, Miller CG, Shively JE, Heinzmann C, Sparkes RS, Mohandas T (1988) Hormone-sensitive lipase: sequence, expression, and chromosomal localization to 19 cent-q13.3. Science 241:1503–1506
Holm C, Osterlund T, Laurell H, Contreras JA (2000) Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annu Rev Nutr 20:365–393
Hung SSO, Wei L, Hongbin L, Storebakken T, Yibo C (1997) Effect of starvation on some morphological and biochemical parameters in white sturgeon (Acipenser transm on tanus). Aquaculture 151:357–363
Jobling M (2006) Effects of starvation on proximate chemical composition and energy utilization of plaice, Pleuronectes platessa L. J Fish Biol 17:325–334
Joon YK, Francisco P, Clive R, Charles RT (2001) Tyler molecular characterization of putative yolk processing enzymes and their expression during oogenesis and embryogenesis in rainbow trout (Oncorhynchus mykiss). Biol Reprod 65:1701–1709
Kraemer FB, Shen WJ (2002) Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res 43:1585–1594
Kraemer FB, Patel S, Saedi MS, Sztalryd C (1993) Detection of hormone-sensitive lipase in various tissues I. Expression of an HSL/bacterial fusion protein and generation of anti-HSL antibodies. J Lipid Res 34:663–671
Ladu MJ, Kapsas H, Palmer WK (1991) Regulation of lipoprotein lipase in adipose and muscle tissues during fasting. Am J Physiol 260:R953–R959
Langfort J, Ploug T, Ihlemann J, Saldo M, Holm C, Galbo H (1999) Expression of hormone-sensitive lipase and its regulation by adrenaline in skeletal muscle. Biochem J 340:459–465
Liang XF, Oku H, Ogata HY (2002) The effects of feeding condition and dietary lipid level on lipoprotein lipase gene expression in liver and visceral adipose tissue of red sea bream Pagrus major. Comp Biochem Physiol A Mol Integr Physiol 131:335–420
Lindberg A, Olivecrona G (2002) Lipoprotein lipase from rainbow trout differs in several respects from the enzyme in mammals. Gene 292:213–223
Londos C, Brasaemle DL, Schultz CJ, Adler-Wailes DC, Levin DM, Kimmel AR, Rondinone CM (1999) On the control of lipolysis in adipocytes. Ann N Y Acad Sci 892:155–168
Luo Z, Liu YJ, Mai KS, Tian LX, Tan XY, Shi JF (2006) Effects of feeding levels on growth performance, feed utilization, body composition, and apparent digestibility coefficients of nutrients for grouper Epinephelus coioides juveniles. J World Aquacult Soc 37:32–40
Luo Z, Tan XY, Wang WM, Fan QX (2009) Effects of long-term starvation on body weight and body composition of juvenile channel catfish, Ictalurus punctatus, with special emphasis on amino acid and fatty acid changes. J Appl Ichthyol 25:184–189
Navarro I, Gutiérrez J (1995) Fasting and starvation. In: Hochachka PW, Mommsen TP (eds) Biochemistry and molecular biology of fishes, vol 4. Elsevier, New York, pp 393–433
Ong JM, Simsolo RB, Saghizadeh M, Pauer A, Kern PA (1994) Expression of lipoprotein lipase in rat muscle: regulation by feeding and hypothyroidism. J Lipid Res 35:1542–1552
Peinado-Onsurbe J, Staels B, Deeb S, Ramirez I, Llobera M, Auwerx J (1992) Neonatal extinction of liver lipoprotein lipase expression. Biochim Biophys Acta 1131:281–286
Reinitz G (1983) Relative effect of age, diet, and feeding rate on the body composition of young rainbow trout (Salmo gairdneri). Aquaculture 35:19–27
Salem M, Silverstein J, Rexroad CE, Yao J (2007) Effect of starvation on global gene expression and proteolysis in rainbow trout (Oncorhynchus mykiss). BMC Genomics 8:328
Sato K, Akiba Y (2002) Lipoprotein lipase mRNA expression in abdominal adipose tissue is little modified by age and nutritional state in broiler chickens. Poult Sci 81:846–852
Sato K, Akiba Y, Kimura S, Horiguchi M (1995) Species differences between chicks and rats inhibition of lipoprotein hydrolysis by Triton WR-1339. Comp Biochem Physiol 112C:315–319
Semenkovich CF, Chen SH, Wims M, Luo CC, Li WH, Chan L (1989) Lipoprotein lipase and hepatic lipase mRNA tissue specific expression, development regulations and evolution. J Lipid Res 30:423–431
Shen WJ, Patel S, Natu V, Kraemer FB (1998) Mutational analysis of structural features of rat hormone-sensitive lipase. Biochemistry 37:8973–8979
Slavin BG, Ong JM, Kern PA (1994) Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. J Lipid Res 35:1535–1541
Sorisky A (1999) From preadipocyte to adipocyte: differentiation-directed signals of insulin from the cell surface to the nucleus. Crit Rev Clin Lab Sci 36:1–34
Spurlock ME, Ji SQ, Godat RL, Kuske JL, Willis GM, Frank GR, Cornelius SG (2001) Changes in the expression of uncoupling proteins and lipases in porcine adipose tissue and skeletal muscle during feed deprivation. J Nutr Biochem 12:81–87
Sugden MC, Holness MJ, Howard RM (1993) Changes in lipoprotein lipase activities in adipose tissue, heart and skeletal muscle during continuous or interrupted feeding. Biochem J 292:113–119
Sztalryd C, Kraemer FB (1994) Regulation of hormone sensitive lipase during fasting. Am J Physiol 266:E179–E185
Sztalryd C, Kraemer FB (1995) Regulation of hormone-sensitive lipase in streptozotocin-treated rats. Metabolism 44:1391–1396
Valente LMP, Gomes EFS, Fauconneau B (1998) Biochemical growth characterization of fast and slow-growing rainbow trout strains: effect of cell proliferation and size. Fish Physiol Biochem 18:213–224
Wilson BE, Deeb S, Florant GL (1992) Seasonal changes in hormone-sensitive and lipoprotein lipase messenger RNA concentrations in marmot white adipose tissue. Am J Physiol 262:R177–R181
Zhang SL, Gu RB, Xu GC, Wen HB (2006) Effect of starvation on the chief biochemical indicator in muscle and serum of Flower Xenocypris. J Shanghai Jiaotong Univ 6:513–516 (Edition of agricultural science) (Chinese version)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, C., Wen, X., Zheng, Q. et al. Effect of starvation on activities and mRNA expression of lipoprotein lipase and hormone-sensitive lipase in tilapia (Oreochromis niloticus × O. areus). Fish Physiol Biochem 37, 113–122 (2011). https://doi.org/10.1007/s10695-010-9423-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-010-9423-6