Abstract
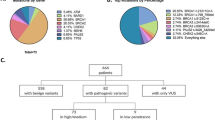
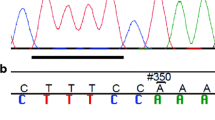
Germline mutations in BRCA1 and BRCA2 account for ~30 % of inherited breast cancer. BRIP1 and PALB2 are likely genes for breast cancer susceptibility, based on their roles in maintaining cellular integrity. Indeed, few pathogenic germline mutations in both genes are reported in ethnically diverse breast cancer families. There is a paucity of data on the putative contribution of both genes to inherited breast cancer in Jewish high risk families. High risk Jewish women, none of whom was a carrier of the predominant Jewish mutations in BRCA1/BRCA2, were screened for BRIP1 germline mutations by combined denaturing gradient gel electrophoresis, high resolution melting and sequencing. Direct sequencing of exons and flanking intronic sequences was used for PALB2 mutational analysis. Overall, 149 women, all of high risk, cancer prone families of Ashkenazi origin, were genotyped for BRIP1 mutations: 127 with breast cancer, 22 with ovarian cancer. No truncating mutations were noted and one novel (p.Ala745Thr) and two previously described missense mutations were detected. For PALB2, 93 women were genotyped (87 with breast cancer) of Ashkenazi (n = 32) and non Ashkenazi Jewish origin. Fifteen sequence variants were detected, of these, none was truncating, four were not previously reported, and two (p.Asp871Gly and p.Leu1119Pro) were seemingly pathogenic based on the PolyPhen2 protein prediction algorithm. These missense mutations were not detected in any of 113 healthy Ashkenazi and 109 Moroccan, cancer free controls. In conclusion, germline mutations in BRIP1 and PALB2 contribute marginally to breast cancer susceptibility in ethnically diverse, Jewish high risk families.
Similar content being viewed by others
References
Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tulinius H, Thorlacius S, Eerola H, Nevanlinna H, Syrjäkoski K, Kallioniemi OP, Thompson D, Evans C, Peto J, Lalloo F, Evans DG, Easton DF (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 72(5):1117–1130
Anglian Breast Cancer Study Group (2000) Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br J Cancer 83:1301–1308
Irving M, Elmslie F, Berg J (2002) Genetics of breast cancer. Int J Clin Pract 56:677–682
Thompson D, Easton D (2004) The genetic epidemiology of breast cancer genes. J Mammary Gland Biol Neoplasia 9:221–236
Kitao H, Nanda I, Sugino RP, Kinomura A, Yamazoe M, Arakawa H, Schmid M, Innan H, Hiom K, Takata M (2011) FancJ/Brip1 helicase protects against genomic losses and gains in vertebrate cells. Genes Cells 16(6):714–727
Cantor SB, Guillemette S (2011) Hereditary breast cancer and the BRCA1-associated FANCJ/BACH1/BRIP1. Future Oncol 7(2):253–261
Tischkowitz M, Xia B (2010) PALB2/FANCN: recombining cancer and Fanconi anemia. Cancer Res 70(19):7353–7359
Tischkowitz M, Xia B, Sabbaghian N, Reis-Filho JS, Hamel N, Li G, van Beers EH, Li L, Khalil T, Quenneville LA, Omeroglu A, Poll A, Lepage P, Wong N, Nederlof PM, Ashworth A, Tonin PN, Narod SA, Livingston DM, Foulkes WD (2007) Analysis of PALB2/FANCN-associated breast cancer families. Proc Natl Acad Sci U S A 104(16):6788–6793
Seal S, Thompson D, Renwick A, Elliott A, Kelly P, Barfoot R, Chagtai T, Jayatilake H, Ahmed M, Spanova K, North B, McGuffog L, Evans DG, Eccles D, Easton DF, Stratton MR, Rahman N (2006) Truncating mutations in the Fanconi anemia J gene BRIP1 are low-penetrance breast cancer susceptibility alleles. Nat Genet 38(11):1239–1241
Wong MW, Nordfors C, Mossman D, Pecenpetelovska G, Avery-Kiejda KA, Talseth-Palmer B, Bowden NA, Scott RJ (2011) BRIP1, PALB2, and RAD51C mutation analysis reveals their relative importance as genetic susceptibility factors for breast cancer. Breast Cancer Res Treat 127(3):853–859
Rohlfs EM, Learning WG, Friedman KJ, Couch FJ, Weber BL, Silverman LM (1997) Direct detection of mutations in the breast and ovarian cancer susceptibility gene BRCA1 by PCR-mediated site-directed mutagenesis. Clin Chem 43:24–29
Reid S, Schindler D, Hanenberg H, Barker K, Hanks S, Kalb R, Neveling K, Kelly P, Seal S, Freund M, Wurm M, Batish SD, Lach FP, Yetgin S, Neitzel H, Ariffin H, Tischkowitz M, Mathew CG, Auerbach AD, Rahman N (2007) Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat Genet 39:162–164
De Nicolo A, Tancredi M, Lombardi G, Flemma CC, Barbuti S, Di Cristofano C, Sobhian B, Bevilacqua G, Drapkin R, Caligo MA (2008) A novel breast cancer-associated BRIP1 (FANCJ/BACH1) germ-line mutation impairs protein stability and function. Clin Cancer Res 14(14):4672–4680
Lewis AG, Flanagan J, Marsh A, Pupo GM, Mann G, Spurdle AB, Lindeman GJ, Visvader JE, Brown MA, Chenevix-Trench G, Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (2005) Mutation analysis of FANCD2, BRIP1/BACH1, LMO4 and SFN in familial breast cancer. Breast Cancer Res 7(6):R1005–R1016
Kuusisto KM, Bebel A, Vihinen M, Schleutker J, Sallinen SL (2011) Screening for BRCA1, BRCA2, CHEK2, PALB2, BRIP1, RAD50, and CDH1 mutations in high-risk Finnish BRCA1/2-founder mutation-negative breast and/or ovarian cancer individuals. Breast Cancer Res 13(1):R20–R32
Guénard F, Labrie Y, Ouellette G, Joly Beauparlant C, Simard J, Durocher F; INHERIT BRCAs. Collaborators (7) Bessette P, Chiquette J, Laframboise R, Lepine J, Lesperance B, Pichette R, Plante M (2008) Mutational analysis of the breast cancer susceptibility gene BRIP1/BACH1/FANCJ in high-risk non-BRCA1/BRCA2 breast cancer families. J Hum Genet 53(7):579–591
García MJ, Fernández V, Osorio A, Barroso A, Llort G, Lázaro C, Blanco I, Caldés T, de la Hoya M, Ramón Y, Cajal T, Alonso C, Tejada MI, San Román C, Robles-Díaz L, Urioste M, Benítez J (2009) Analysis of FANCB and FANCN/PALB2 fanconi anemia genes in BRCA1/2-negative Spanish breast cancer families. Breast Cancer Res Treat 113(3):545–551
Rutter JL, Smith AM, Dávila MR, Sigurdson AJ, Giusti RM, Pineda MA, Doody MM, Tucker MA, Greene MH, Zhang J, Struewing JP (2003) Mutational analysis of the BRCA1-interacting genes ZNF350/ZBRK1 and BRIP1/BACH1 among BRCA1 and BRCA2-negative probands from breast-ovarian cancer families and among early-onset breast cancer cases and reference individuals. Hum Mutat 22(2):121–128
Cao AY, Huang J, Hu Z, Li WF, Ma ZL, Tang LL, Zhang B, Su FX, Zhou J, Di GH, Shen KW, Wu J, Lu JS, Luo JM, Yuan WT, Shen ZZ, Huang W, Shao ZM (2009) Mutation analysis of BRIP1/BACH1 in BRCA1/BRCA2 negative Chinese women with early onset breast cancer or affected relatives. Breast Cancer Res Treat 115(1):51–55
Silvestri V, Rizzolo P, Falchetti M, Zanna I, Masala G, Bianchi S, Palli D, Ottini L (2011) Mutation analysis of BRIP1 in male breast cancer cases: a population-based study in Central Italy. Breast Cancer Res Treat 126(2):539–543
Rafnar T, Gudbjartsson DF, Sulem P, Jonasdottir A, Sigurdsson A, Jonasdottir A, Besenbacher S, Lundin P, Stacey SN, Gudmundsson J, Magnusson OT, le Roux L, Orlygsdottir G, Helgadottir HT, Johannsdottir H, Gylfason A, Tryggvadottir L, Jonasson JG, de Juan A, Ortega E, Ramon-Cajal JM, García-Prats MD, Mayordomo C, Panadero A, Rivera F, Aben KK, van Altena AM, Massuger LF, Aavikko M, Kujala PM, Staff S, Aaltonen LA, Olafsdottir K, Bjornsson J, Kong A, Salvarsdottir A, Saemundsson H, Olafsson K, Benediktsdottir KR, Gulcher J, Masson G, Kiemeney LA, Mayordomo JI, Thorsteinsdottir U, Stefansson K (2011) Mutations in BRIP1 confer high risk of ovarian cancer. Nat Genet 43(11):1104–1107
Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, Roeb W, Agnew KJ, Stray SM, Wickramanayake A, Norquist B, Pennington KP, Garcia RL, King MC, Swisher EM (2011) Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A 108(44):18032–18037
Casadei S, Norquist BM, Walsh T, Stray S, Mandell JB, Lee MK, Stamatoyannopoulos JA, King MC (2011) Contribution of inherited mutations in the BRCA2-interacting protein PALB2 to familial breast cancer. Cancer Res 71(6):2222–2229
Stadler ZK, Salo-Mullen E, Sabbaghian N, Simon JA, Zhang L, Olson SH, Kurtz R, Offit K, Foulkes WD, Robson ME, Tischkowitz M (2011) Germline PALB2 mutation analysis in breast-pancreas cancer families. J Med Genet 48(8):523–525
Pylkäs K, Erkko H, Nikkilä J, Sólyom S, Winqvist R (2008) Analysis of large deletions in BRCA1, BRCA2 and PALB2 genes in Finnish breast and ovarian cancer families. BMC Cancer 8:146–150
Solyom S, Pylkäs K, Winqvist R (2010) Screening for large genomic rearrangements of the BRIP1 and CHK1 genes in Finnish breast cancer families. Fam Cancer 9(4):537–540
Blanco A, de la Hoya M, Balmaña J, Ramón Y, Cajal T, Teulé A, Miramar MD, Esteban E, Infante M, Benítez J, Torres A, Tejada MI, Brunet J, Graña B, Balbín M, Pérez-Segura P, Osorio A, Velasco EA, Chirivella I, Calvo MT, Feliubadaló L, Lasa A, Díez O, Carracedo A, Caldés T, Vega A (2012) Detection of a large rearrangement in PALB2 in Spanish breast cancer families with male breast cancer. Breast Cancer Res Treat 132(1):307–315
Zheng Y, Zhang J, Niu Q, Huo D, Olopade OI (2011) Novel germline PALB2 truncating mutations in African American breast cancer patients. Cancer doi: 10.1002/cncr.26388. [Epub ahead of print]
Hellebrand H, Sutter C, Honisch E, Gross E, Wappenschmidt B, Schem C, Deissler H, Ditsch N, Gress V, Kiechle M, Bartram CR, Schmutzler RK, Niederacher D, Arnold N, Meindl A (2011) Germline mutations in the PALB2 gene are population specific and occur with low frequencies in familial breast cancer. Hum Mutat 32(6):E2176–E2188
Hofstatter EW, Domchek SM, Miron A, Garber J, Wang M, Componeschi K, Boghossian L, Miron PL, Nathanson KL, Tung N (2011) PALB2 mutations in familial breast and pancreatic cancer. Fam Cancer 10(2):225–231
Peterlongo P, Catucci I, Pasquini G, Verderio P, Peissel B, Barile M, Varesco L, Riboni M, Fortuzzi S, Manoukian S, Radice P (2011) PALB2 germline mutations in familial breast cancer cases with personal and family history of pancreatic cancer. Breast Cancer Res Treat 126(3):825–828
Southey MC, Teo ZL, Dowty JG, Odefrey FA, Park DJ, Tischkowitz M, Sabbaghian N, Apicella C, Byrnes GB, Winship I, Baglietto L, Giles GG, Goldgar DE, Foulkes WD, Hopper JL, kConFab for the Beast Cancer Family Registry (2010) A PALB2 mutation associated with high risk of breast cancer. Breast Cancer Res 12(6):R109–R118
Bogdanova N, Sokolenko AP, Iyevleva AG, Abysheva SN, Blaut M, Bremer M, Christiansen H, Rave-Fränk M, Dörk T, Imyanitov EN (2011) PALB2 mutations in German and Russian patients with bilateral breast cancer. Breast Cancer Res Treat 126(2):545–550
Ding YC, Steele L, Chu LH, Kelley K, Davis H, John EM, Tomlinson GE, Neuhausen SL (2011) Germline mutations in PALB2 in African-American breast cancer cases. Breast Cancer Res Treat 126(1):227–230
Ding YC, Steele L, Kuan CJ, Greilac S, Neuhausen SL (2011) Mutations in BRCA2 and PALB2 in male breast cancer cases from the United States. Breast Cancer Res Treat 126(3):771–778
Balia C, Sensi E, Lombardi G, Roncella M, Bevilacqua G, Caligo MA (2010) PALB2: a novel inactivating mutation in a Italian breast cancer family. Fam Cancer 9(4):531–536
Guénard F, Pedneault CS, Ouellette G, Labrie Y, Simard J, INHERIT, Durocher F (2010) Evaluation of the contribution of the three breast cancer susceptibility genes CHEK2, STK11, and PALB2 in non-BRCA1/2 French Canadian families with high risk of breast cancer. Genet Test Mol Biomarkers 14(4):515–526
Adank MA, van Mil SE, Gille JJ, Waisfisz Q, Meijers-Heijboer H (2011) PALB2 analysis in BRCA2-like families. Breast Cancer Res Treat 127(2):357–362
Silvestri V, Rizzolo P, Zanna I, Falchetti M, Masala G, Bianchi S, Papi L, Giannini G, Palli D, Ottini L (2010) PALB2 mutations in male breast cancer: a population-based study in Central Italy. Breast Cancer Res Treat 122(1):299–301
Dansonka-Mieszkowska A, Kluska A, Moes J, Dabrowska M, Nowakowska D, Niwinska A, Derlatka P, Cendrowski K, Kupryjanczyk J (2010) A novel germline PALB2 deletion in Polish breast and ovarian cancer patients. BMC Med Genet 11:20–28
Ghadirian P, Robidoux A, Zhang P, Royer R, Akbari M, Zhang S, Fafard E, Costa M, Martin G, Potvin C, Patocskai E, Larouche N, Younan R, Nassif E, Giroux S, Narod SA, Rousseau F, Foulkes WD (2009) The contribution of founder mutations to early-onset breast cancer in French-Canadian women. Clin Genet 76(5):421–426
Papi L, Putignano AL, Congregati C, Piaceri I, Zanna I, Sera F, Morrone D, Genuardi M, Palli D (2010) A PALB2 germline mutation associated with hereditary breast cancer in Italy. Fam Cancer 9(2):181–185
Sluiter M, Mew S, van Rensburg EJ (2009) PALB2 sequence variants in young South African breast cancer patients. Fam Cancer 8(4):347–353
Gunnarsson H, Arason A, Gillanders EM, Agnarsson BA, Johannesdottir G, Johannsson OT, Barkardottir RB (2008) Evidence against PALB2 involvement in Icelandic breast cancer susceptibility. J Negat Results Biomed 7:5–8
Cao AY, Huang J, Hu Z, Li WF, Ma ZL, Tang LL, Zhang B, Su FX, Zhou J, Di GH, Shen KW, Wu J, Lu JS, Luo JM, Yuan WT, Shen ZZ, Huang W, Shao ZM (2009) The prevalence of PALB2 germline mutations in BRCA1/BRCA2 negative Chinese women with early onset breast cancer or affected relatives. Breast Cancer Res Treat 114(3):457–462
Foulkes WD, Ghadirian P, Akbari MR, Hamel N, Giroux S, Sabbaghian N, Darnel A, Royer R, Poll A, Fafard E, Robidoux A, Martin G, Bismar TA, Tischkowitz M, Rousseau F, Narod SA (2007) Identification of a novel truncating PALB2 mutation and analysis of its contribution to early-onset breast cancer in French-Canadian women. Breast Cancer Res 9(6):R83–R91
Erkko H, Xia B, Nikkilä J, Schleutker J, Syrjäkoski K, Mannermaa A, Kallioniemi A, Pylkäs K, Karppinen SM, Rapakko K, Miron A, Sheng Q, Li G, Mattila H, Bell DW, Haber DA, Grip M, Reiman M, Jukkola-Vuorinen A, Mustonen A, Kere J, Aaltonen LA, Kosma VM, Kataja V, Soini Y, Drapkin RI, Livingston DM, Winqvist R (2007) A recurrent mutation in PALB2 in Finnish cancer families. Nature 446(7133):316–319
Acknowledgments
We thank all individuals who participated to this study. This study was partially funded by Italian citizens who allocated the 5 × 1000 share of their tax payment in support of the Fondazione IRCCS Istituto Nazionale Tumori, according to Italian laws (INT-Institutional strategic projects ‘5 × 1000’). This study was partuially funded by the Israel Cancer Association. This work was done in partial fulfillment of the requirements for a Masters degree for Roni Milgrom and Anya Kushnir at the Department of Human Genetics and Molecular Biology at the Tel-Aviv University, Tel-Aviv, Israel.
Conflict of interest
All authors declare that they have no conflict of interest regarding the data published herein.
Author information
Authors and Affiliations
Corresponding author
Additional information
Irene Catucci, Roni Milgrom, Eitan Friedman and Paolo Peterlongo have equally contributed to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Catucci, I., Milgrom, R., Kushnir, A. et al. Germline mutations in BRIP1 and PALB2 in Jewish high cancer risk families. Familial Cancer 11, 483–491 (2012). https://doi.org/10.1007/s10689-012-9540-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-012-9540-8