Abstract
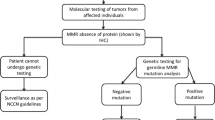
Lynch syndrome (LS), or hereditary non-polyposis colorectal cancer (HNPCC), is an autosomal dominant condition responsible for early onset cancer mostly in the colonrectum and endometrium as well as in other organ sites. Lynch syndrome is caused by germline mutations in mismatch repair genes, prevalently in hMSH2, hMLH1, and less frequently in hMSH6 and hPMS2. Twenty-nine non-related index cases with colorectal cancer (CRC) were collected from a region in southeast Italy (Apulia). Among this set of patients, fifteen fulfilled the Amsterdam criteria II. The presence of tumor microsatellite instability (MSI) was assessed in all index cases and 19 (15 AC+/4 AC−) were classified as MSI-H. Mutation analysis performed on all patients, identified 15 pathogenic mutations in hMLH1 and 4 in hMSH2. 4/15 mutations in hMLH1 and 2/4 hMSH2 mutations have not been previously reported. Three previously reported mutations were further investigated for the possibility of a common founder effect. Genetic counseling was offered to all probands and extended to 183 relatives after molecular testing and 85 (46%) mutation carriers were identified. Eighty mutation carriers underwent an accurate clinical and instrumental surveillance protocol. Our results confirm that the identification of LS patients based exclusively on family history may miss patients carrying germline mutations in the MMR genes. Moreover, our results demonstrated that molecular screening and subsequent instrumental surveillance are very effective in identifying CRCs at earlier stages and reducing the number of deaths from secondary cancers in HNPCC patients.



Similar content being viewed by others
Abbreviations
- HNPCC:
-
Hereditary non polyposis colorectal cancer
- MMR:
-
Mismatch repair
- CRC:
-
Colorectal cancer
- LS:
-
Lynch syndrome
- MSI:
-
Microsatellite instability
- AC:
-
Amsterdam criteria
- BG:
-
Bethesda guidelines
- RBG:
-
Revised Bethesda guidelines
- IHC:
-
Immunohistochemistry
- MTS:
-
Muir-Torre syndrome
- DHPLC:
-
Denaturing high pressure liquid chromatography
- MLPA:
-
Multiplex ligation-dependent probe amplification
- InSIGHT:
-
International society for gastrointestinal hereditary tumors
References
Hampel H, Frankel Wl, Martin E et al (2005) Screening for the lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 352:1851–1860
Lynch H, de la Chapelle A (2003) Hereditary colorectal cancer. N Engl J Med 348(10):919–932
Peltomäki P, Vasen HF (1997) Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborative study. The International Collaborative Group on hereditary nonpolyposis colorectal cancer. Gastroenterology 11:1146–1158
Lagerstedt Robinson K, Liu T, Vandrovcova J et al (2007) Lynch syndrome (hereditary nonpolyposis colorectal cancer) diagnostics. J Natl Cancer Inst 99(4):291–299
Mueller J, Gazzoli I, Bandipalliam P, Garber JE, Syngal S, Kolodner RD (2009) Comprehensive molecular analysis of mismatch repair gene defects in suspected Lynch syndrome (hereditary nonpolyposis colorectal cancer) cases. Cancer Res 69:7053–7061
n Cappel WH, Nagengast FM, Griffioen G (2002) Surveillance for hereditary nonpolyposis colorectal cancer: a long-term study on 114 families. Dis Colon Rectum 45(12):1588–1594
Lynch HT (1996) Is there a role for prophylactic subtotal colectomy among hereditary nonpolyposis colorectal cancer germline mutation carriers? Dis Colon Rectum 39(1):109–110
Perea J, Justo I, Alvaro M (2009) Surgical management of hereditary colorectal cancer surgery based on molecular analysis and family history. Rev Esp Enferm Dig 101(8):536–540
Lindor NM, Petersen GM, Hadley DW et al (2006) Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA 296(12):1507–1517
Jarvinen HJ, Aarnio M, Mustonen H (2000) Controlled 15-years trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 118(5):829–834
Vasen HF, Mecklin JP, Khan PM, Lynch HT (1991) The International Collaborative Group on hereditary nonpolyposis colorectal cancer (ICG-HNPCC). Dis Col Rectum 34(5):424–425
Rodriguez-Bigas MA, Boland CR, Hamilton SR (1997) A National Cancer Institute workshop on hereditary non polyposis colorectal cancer syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst 89(22):1758–1762
Umar A, Boland CR, Terdiman JP et al (2004) Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96(4):261–268
Hampel H, Frankel WL, Martin E (2008) Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol 26(35):5783–5788
Barnetson RA, Tenesa A, Farrington S (2006) Identification and survival of carriers of mutations in DNA Mismatch-repair genes in colon cancer. New Engl J Med 354(26):2751–2761
Kastrinos F, Steyerberg EW, Mercado R et al. (2010) The PREMM 1,2,6 Model predicts risk of MLH1, MSH2, and MSH6 Germline Mutations based on cancer history. Gastroenterology Aug 18. [Epub ahead of print]
Boland CR, Thibodeau SN, Hamilton SR (1998) A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58(22):5248–5257
Pedroni M, Sala E, Scarselli A et al (2001) Microsatellite instability and mismatch-repair protein expression in hereditary and sporadic colorectal carcinogenesis. Cancer Res 61:896–899
Holinski-Feder E, Muller-Koch Y, Friedl W (2001) DHPLC mutation analysis of the hereditary nonpolyposis colon cancer (HNPCC) genes hMLH1 and hMSH2. J Biochem Biophys Methods 47(1–2):21–32
Lastella P, Surdo NC, Resta N, Guanti G, Stella A (2006) In silico and in vivo splicing analysis of MLH1 and MSH2 missense mutations shows exon- and tissue-specific effects. BMC Genomics 7:243
den Dunnen JT, Antonarakis SE (2000) Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat 15(1):7–12
Stella A, Surdo NC, Lastella P et al (2007) Germline novel MSH2 deletions and a founder MSH2 deletion associated with anticipation effects in HNPCC. Clin Genet 71(2):130–139
Tournier I, Vezain M, Martins A et al (2008) A large fraction of unclassified variants of the mismatch repair genes MLH1 and MSH2 is associated with splicing defects. Hum Mutat 29:1412–1424
Piepoli A, Santoro R, Cristofaro G et al (1996) Linkage analysis identifies gene carriers among members of families with hereditary nonpolyposis colorectal cancer. Gastroenterology 110(5):1404–1409
Viel A, Genuardi M, Lucci-Cordisco E et al (1998) Hereditary nonpolyposis colorectal cancer: an approach to the selection of candidates to genetic testing based on clinical and molecular characteristics. Community Genet 1(4):229–236
Benatti P, Roncucci L, Percesepe A et al (1998) Small bowel carcinoma in hereditary nonpolyposis colorectal cancer. Am J Gastroenterol 93(11):2219–2222
Wagner A, Tops C, Wijnen JT et al (2002) Genetic testing in hereditary non-polyposis colorectal cancer families with a MSH2, MLH1, or MSH6 mutation. J Med Genet 39:833–837
Julié C, Trésallet C, Brouquet A et al (2008) Identification in daily practice of patients with Lynch syndrome (hereditary nonpolyposis colorectal cancer): revised Bethesda guidelines-based approach versus molecular screening. Am J Gastroenterol 103:2825–2835
Wijnen J, Vasen H, Khan PM et al (1995) Seven new mutations in hMSH2, an HNPCC gene, identified by denaturing gradient-gel electrophoresis. Am J Hum Genet 56(5):1060–1066
Wagner A, Barrows A, Wijnen JT et al (2003) Molecular analysis of hereditary nonpolyposis colorectal cancer in the United States: high mutation detection rate among clinically selected families and characterization of an American founder genomic deletion of the MSH2 gene. Am J Hum Genet 72(5):1088–1100
Desai DC, Lockman JC, Chadwick RB et al (2000) Recurrent germline mutation in MSH2 arises frequently de novo. J Med Genet 37(9):646–652
Foulkes WD, Thiffault I, Gruber SB et al (2002) The founder mutation MSH2*1906G>C in an important cause of hereditary nonpolyposis colorectal cancer in the Ashkenazi Jewish population. Am J Hum Genet 71(6):1395–1412
Caluseriu O, Di Gregorio C, Lucci-Cordisco E et al (2004) A founder MLH1 mutation in families from the districts of Modena and Reggio-Emilia in northern Italy with hereditary non-polyposis colorectal cancer associated with protein elongation and instability. J Med Genet 41(3):e34
Chan TL, Chan YW, Ho JW et al (2004) MSH2 c.1452–1455delAATG is a founder mutation and an important cause of hereditary nonpolyposis colorectal cancer in the southern Chinese population. Am J Hum Genet 74(5):1035–1042
Pensotti V, Radice P, Presciutini S et al (1997) Mean age of tumor onset in hereditary nonpolyposis colorectal cancer (HNPCC) families correlates with the presence of mutations in DNA mismatch repair genes. Genes Chromosomes Cancer 19(3):135–142
Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR (2009) Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis and medico legal ramifications. Clin Genet 76(1):1–18
Kastrinos F, Stoffel EM, Balmaña J, Steyerberg EW, Mercado R, Syngal S (2008) Phenotype comparison of MLH1 and MSH2 mutation carriers in a cohort of 1914 individuals undergoing clinical genetic testing in the United States. Cancer Epidemiol Biomarkers Prev 17(8):2044–2051
n Cappel WH, Nagengast FM, Griffioen G et al (2002) Surveillance for hereditary nonpolyposis colorectal cancer: a long-term study on 114 families. Dis Colon Rectum 45(12):1558–1594
Pylvanainen K, Kairaluoma M, Mecklin JP (2006) Compliance and satisfaction with long-term surveillance in Finnish HNPCC families. Fam Cancer 5(2):175–178
Järvinen HJ, Renkonen-Sinisalo L, Aktán-Collán K, Peltomäki P, Aaltonen LA, Mecklin JP (2009) Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J Clin Oncol 27:4793–4797
De Jong AE, Morreau H, Van Puijenbroek M et al (2004) The role of mismatch repair gene defects in the development of adenomas in patients with HNPCC. Gastroenterology 126:42–48
German HNPCC Consortium, Müller A, Beckmann C, Westphal G et al (2006) Prevalence of the mismatch-repair-deficient phenotype in colonic adenomas arising in HNPCC patients: results of a 5-year follow-up study. Int J Colorectal Dis 21:632–641
Hurlstone DP, Karajeh M, Cross SS et al (2005) The role of high-magnification-chromoscopic colonoscopy in hereditary nonpolyposis colorectal cancer screening: a prospective “back-to-back” endoscopy study. Am J Gastroenterology 100:2167–2173
Koornstra JJ, Mourits MJ, Sijmons RH et al (2009) Management of extracolonic tumours in patients with Lynch syndrome. Lancet 10(4):400–408
Hüneburg R, Lammert F, Rabe C et al (2009) Chromocolonoscopy detects more adenomas than white light colonoscopy or narrow band imaging colonoscopy in hereditary nonpolyposis colorectal cancer screening. Endoscopy 41(4):316–322
Rex DK, Cutler CS, Lemmel GT et al (1997) Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 112:24–28
Trojan J, Zeuzem S, Randolph A et al (2002) Functional analysis of hMLH1 variants and HNPCC-related mutations using a human expression system. Gastroenterology 122(1):211–219
Takahashi M, Shimodaira H, Andreutti-Zaugg C, Iggo R, Kolodner RD, Ishioka C (2007) Functional analysis of human MLH1 variants using yeast and in vitro mismatch repair assays. Cancer Res 67(10):4595–4604
Raevaara TE, Korhonen MK, Lohi H et al (2005) Functional significance and clinical phenotype of nontruncating mismatch repair variants of MLH1. Gastroenterology 129(2):537–549
Pagenstecher C, Wehner M, Friedl W et al (2006) Aberrant splicing in MLH1 and MSH2 due to exonic and intronic variants. Hum Genet 119(1–2):9–22
Auclair J, Busine MP, Navarro C et al (2006) Systematic mRNA analysis for the effect of MLH1 and MSH2 missense and silent mutations on aberrant splicing. Hum Mutat 27(2):145–154
Kondo E, Suzuki H, Horii A, Fukushige S (2003) A yeast two-hybrid assay provides a simple way to evaluate the vast majority of hMLH1 germline mutations. Cancer Res 63(12):3302–3308
Acknowledgments
Supported by grants: (a) Regione Puglia contract PE 041CARSO Ricerca Oncologica; (b) Fondazione Cassa di Risparmio di Puglia “Identificazione di eventi di splicing associati ai tumori del colon retto” and (c) Ricerca Finalizzata Università di Bari (60%) all to AS. The authors wish to thank Clare Hannon (Bsc Hons) and Gennaro Lenato (PhD) for their careful editing and critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lastella, P., Patruno, M., Forte, G. et al. Identification and surveillance of 19 Lynch syndrome families in southern Italy: report of six novel germline mutations and a common founder mutation. Familial Cancer 10, 285–295 (2011). https://doi.org/10.1007/s10689-011-9419-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-011-9419-0