Abstract
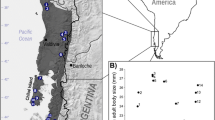
According to Bergmann’s rule, in endothermic species, body sizes of individuals tend to be larger in colder climates compared to those in warmer climates. Some ectotherms, including amphibians, have also been found to conform to this rule. However, the validity of this rule is disputed, as it is uncertain whether Bergmann’s clines are generally applicable to all anuran species. Here we studied altitudinal variation in mean body size, egg size, age, and growth rate in Bufo minshanicus across six altitudes in the eastern Tibetan Plateau. Our results showed that mean body size increased with increasing altitude, which is consistent with Bergmann’s rule. Toads from higher altitudes also tended to have faster growth rates and older mean ages of reproductively mature adults, but did not have larger egg sizes. We suggest that, as growth rate is positively correlated with altitude, and it contributes to body size variation more than mean age does, then this explains why this species follows Bergmann’s rule.





Similar content being viewed by others
References
Adams DC, Church JO (2008) Amphibians do not follow Bergmann’s rule. Evolution 62:413–420
Angilletta MJ, Wilson RS, Navas CA et al (2003) Tradeoffs and the evolution of thermal reaction norms. Trends Ecol Evol 18:234–240
Angilletta MJ, Niewiarowski PH, Dunham AE et al (2004) Bergmann’s clines in ectotherms: illustrating a life-history perspective with sceloporine lizards. Am Nat 164:168–183
Ashton KG (2002a) Patterns of within-species body size variation of birds: strong evidence for Bergmann’s rule. Glob Ecol Biogeogr 11:505–523
Ashton KG (2002b) Do amphibians follow Bergmann’s rule? Can J Zool 80:708–716
Ashton KG, Feldman CR (2003) Bergmann’s rule in nonavian reptiles: turtles follow it, lizards and snakes reverse it. Evolution 57:1151–1163
Badyaev AV, Ghalambor CK (2001) Evolution of life histories along elevational gradients: trade-off between parental care and fecundity. Ecology 82:2948–2960
Beck E, Kottke I, Bendix J, Makeschin F, Mosandl R (2008) Gradients in a tropical mountain ecosystem—asynthesis. In: Beck E, Bendix J, Kottke I, Makeschin F, Mosandl R (eds) Gradients in a tropical mountain ecosystem of ecuador. Ecological studies, vol 198. Springer, Berlin, Heidelberg, pp 451–463
Belk MC, Houston DD (2002) Bergmann’s rule in ectotherms: a test using freshwater fishes. Am Nat 160:803–808
Bergmann C (1847) Über die verhältnisse der warmeökonomie der thiere zuihrer grosse. Gott Stud 1:595–708
Berven KA (1982) The genetic basis of altitudinal variation in the wood frog Rana sylvatica. I. An experimental analysis of life history traits. Evolution 36:962–983
Blackburn TM, Hawkins BA (2004) Bergmann’s rule and the mammal fauna of North America. Ecography 27:715–724
Brown JH, Marquet PA, Taper ML (1993) Evolution of body size: consequences of an energetic definition of fitness. Am Nat 142:573–584
Conover DO, Present TMC (1990) Countergradient variation in growth rate: compensation for length of the growing season among Atlantic silversides from different latitudes. Oecologia 83:316–324
de Queiroz A, Ashton KG (2004) The phylogeny of a species-level tendency: species heritability and possible deep origins of Bergmann’s rule in tetrapods. Evolution 58:1674–1684
Dziminski MA, Robert JD (2006) Fitness consequences of variable maternal provisioning in quacking frogs (Crinia georgiana). J Evol Biol 19:144–155
Fei L, Ye CY (2001) The colour handbook of amphibians of Sichuan. China Forestry Publishing House, Beijing
Halliday TR, Verrell PA (1988) Body size and age in amphibians and reptiles. J Herpet 22:253–265
Jin L, Chen C, Liao WB (2017) Altitudinal variation in body size and age in male spot-legged treefrog (Polypedates megacephalus). Russ J Ecol 48:476–481
Kaplan RH, King EG (1997) Egg size is a developmentally plastic trait: evidence from long term studies in the frog Bombina orientalis. Herpetologica 53:149–165
Laugen AT, Laurila A, Räsänen K et al (2003) Latitudinal countergradient variation in the common frog (Rana temporaria) developmental rates-evidence for local adaptation. J Evol Biol 16:996–1005
Laugen AT, Laurila A, Jönsson KI et al (2005) Do common frogs (Rana temporaria) follow Bergmann’s rule? Evol Ecol Res 7:717–731
Levinton JS (1983) The latitudinal compensation hypothesis: growth data and a model of latitudinal growth differentiation based upon energy budgets. I. Interspecific comparison of Ophryotrocha (Polychaeta: Dorvilleidae). Biol Bull-us 165:686–698
Levinton JS, Monahan RK (1983) The latitudinal compensation hypothesis: growth data and a model of latitudinal growth differentiation based upon energy budgets. II. Intraspecific comparisons between subspecies of Ophryotrocha puerilis. Biol Bull 165:699–707
Li ST, Wu X, Li DY, Lou SL, Mi ZP, Liao WB (2013) Body size variation of Odorous Frog (Odorrana grahami) across altitudinal gradients. Herpetol J 23:187–192
Liao WB (2011) A skeletochronlogical estimate of age in a population of the Siberian Wood Frog, Rana amurensis, from northeastern China. Acta Herpetol 6:237–245
Liao WB, Lu X (2011) Variation in body size, age and growth in a subtropical treefrog (Rhacophorus omeimontis) along an altitudinal gradient in western China. Ethol Ecol Evol 23:248–261
Liao WB, Lu X (2012) Adult body size = f(initial size + growth rate × age): explaining the proximate cause of Bergmann’s cline in a toad along altitudinal gradients. Evol Ecol 26:579–590
Liao WB, Zhou CQ, Yang ZS et al (2010a) Age, size and growth in two populations of the dark-spotted frog Rana nigromaculata at different altitudes in southwestern China. Herpetol J 20:77–82
Liao WB, Zhou CQ, Yang ZS, Hu JC, Lu X (2010b) Age, size and growth in two populations of the dark-spotted frog Rana nigromaculata at different altitudes in southwestern China. Herpetol J 20:77–82
Liao WB, Lu X, Shen YW, Hu JC (2011) Age structure and body size of two populations of the rice frog Rana limnocharis from different altitudes. Ital J Zool 78:215–221
Liao WB, Lu X, Jehle R (2014) Altitudinal variation in reproductive investment and trade-off between egg size and clutch size in the Andrew’s Toad (Bufo andrewsi). J Zool 293:84–91
Liao WB, Luo Y, Lou SL, Lu D, Jehle R (2016) Geographic variation in life-history traits: growth season affects age structure, egg size and clutch size in Andrew’s toad (Bufo andrewsi). Front Zool 13:6
Liu Q, Feng H, Jin L, Mi ZP, Zhou ZM, Liao WB (2018) Latitudinal variation in body size in Fejervarya limnocharis supports the inverse of Bergmann’s rule. Anim Biol 68:113–128
Lu X, Li B, Liang JJ (2006) Comparative demography of a temperate anuran, Rana chensinensis, along a relatively fine elevational gradient. Can J Zool 84:1789–1795
Ma XY, Lu X, Merilä J (2009) Altitudinal decline of body size in a Tibetan frog Nanorana parkeri. J Zool 279:364–371
Marangoni F, Tejedo M (2008) Variation in body size and metamorphic traits of Iberian spadefoot toads over a short geographic distance. J Zool 275:97–105
Matthews KR, Miaud C (2007) A skeletochronological study of the age structure, growth, and longevity of a mountain yellow-legged frog, Rana muscosa, in the Sierra Nevada, California. Copeia 2007:986–993
Mayr E (1956) Geographical character gradients and climatic adaptation. Evolution 10:105–108
McCarthy MA, Parris KM (2004) Clarifying the effect of toe clipping on frogs with Bayesian statistics. J Appl Ecol 41:780–786
Miaud C, Guyetant R, Elmberg J (1999) Variations in life-history traits in the common frog Rana temporaria (Amphibia: Anura): a literature review and new data from the French Alps. J Zool 249:61–73
Morrison C, Hero JM (2003) Geographic variation in life-history characteristics of amphibians: a review. J Anim Ecol 72:270–279
Oromi N, Sanuy D, Sinsch U (2012) Altitudinal variation of demographic life-history traits does not mimic latitudinal variation in natterjack toads (Bufo calamita). Zoology 115:30–37
Ryser J (1996) Comparative life histories of a low- and a high-elevation population of the common frog Rana temporaria. Amphibia-Reptilia 17:183–195
Sinsch U, Marangoni F, Oromi N et al (2010) Proximate mechanisms determining size variability in natterjack toads. J Zool Lond 281:272–281
Smirina EM (1994) Age determination and longevity in amphibians. Gerontology 40:133–146
Walters RJ, Hassall M (2006) The temperature-size rule in ectotherms: may a general explanation exist after all? Am Nat 167:510–523
Yamahira K, Conover DO (2002) Intra- vs. interspecific latitudinal variation in growth: adaptation to temperature or seasonality? Ecology 83:1252–1262
Yu T, Lu X (2013) Body size variation of four latitudinally-separated populations of a toad species: age and growth rate as the proximate determinants. Integr Zool 8:315–323
Yu TL, Busam M, Wang DL, Chen K (2016) Plasticity of metamorphic traits in a high-altitude toad: interactive effects of food level and temperature. Amphibia-Reptilia 37:33–43
Acknowledgements
We are very grateful to Wen-hua Qi, Yan-Liang Han and Ji-zu Ning for their assistance in the field. We especially thank the parents and uncle of TLY for helping during all phases of the data collection. All animals were collected under the guidelines for animal care in China. Handling and processing of frogs followed approved protocols from Animal Scientific Procedures Act 1988 by the State Department of China. The Gahai-Zecha National Nature Reserve Management Bureau approved this project (approval number GHZCRMB/03-212014), and gave permission for fieldwork. The study was funded by Emergency Management Program of National Natural Science Foundation of China (Grant no. 31741019), Natural Science Foundation of Henan Province of China (Grant no. 182300410019), Foundation for University Key Teacher of Henan province (Grant no. 2016GGJS-098) and Program for Innovative Research Team (in Science and Technology) in universities of Henan Province (Grant no. 17IRTSTHN019). The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, T.L., Wang, D.L., Busam, M. et al. Altitudinal variation in body size in Bufo minshanicus supports Bergmann’s rule. Evol Ecol 33, 449–460 (2019). https://doi.org/10.1007/s10682-019-09984-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-019-09984-1