Abstract
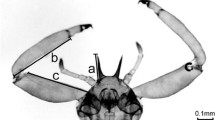
Eusocial aphids produce sterile individuals (“soldiers”) that specialize behaviorally and morphologically to protect their colony from predators, while production of soldiers can negatively affect colony growth because of reproductive allocation and opportunity cost. Hence, a cost-saving soldier production strategy is expected to be favored. Here, we hypothesize that, to save the cost, a eusocial aphid Ceratovacuna japonica produces soldiers with smaller weapon in the season when predators are not abundant. The abundance of two specialist lepidopteran predators (i.e., Taraka hamada and Atkinsonia ignipicta) of C. japonica dramatically increased, and aphid colony size significantly decreased, from July to August. In line with these, the soldiers in August had larger weapons (i.e., frontal horns) than those in June, indicating a correlational increase in weapon size with predation pressure. We predict that a reliable prospective signal indicating the coming of midsummer (environmental temperature) induces mother aphids to produce soldiers with larger weapons. Experiments clarified that soldiers produced at 20 °C (typical temperature of July to August) had larger weapons than those produced at 15 °C (typical temperature of May to July). Such phenotypic plasticity appears to be adaptive to maximize the fitness of C. japonica under a temporally variable but predictable predation environment. These results indicate that C. japonica aphids not merely have distinctive reproductive—and soldier castes, but also produce differentially armed soldiers in a habitat with temporally changing predation risks.







Similar content being viewed by others
References
Agrawal AA (2001) Ecology: phenotypic plasticity in the interactions and evolution of species. Science 294:321–326
Agrawal AA, Ackerly DD, Adler F, Arnold AE, Caceres C, Doak DF, Post E, Hudson PJ, Maron J, Mooney KA, Power M, Schemske D, Stachowicz J, Strauss S, Turner MG, Werner E (2007) Filling key gaps in population and community ecology. Front Ecol Environ 5:145–152
Akimoto S (1992) Shift in life-history strategy from reproduction to defense with colony age in the galling aphid Hemipodaphis persimilis producing defensive first-instar larvae. Res Popul Ecol 34:359–372
Aoki S (1977) Colophina clematis (Homoptera, Pemphigidae), an aphid species with soldiers. Kontyû 45:276–282
Aoki S (1984) Heitai wo motta aburamushi. Doubutsusya press, Tokyo, pp 66–67
Aoki S, Kurosu U (1985) An aphid species doing a headstand: butting behavior of Astegopteryx bambucifoliae (Homoptera: Aphidoidea). J Ethol 3:83–87
Aoki S, Kurosu U (1991) Discovery of the gall generation of Ceratovacuna japonica (Homoptera: Aphidoidea). Akitu 122:1–6
Aoki S, Kurosu U (2011) A review of the biology of Cerataphidini (Hemiptera, Aphididae, Hormaphidinae), focusing mainly on their life cycles, gall formation, and soldiers. Psyche 2010: Article ID 380351
Aoki S, Miyazaki M (1978) Notes on the pseudoscorpion–like larvae of Pseudoregma alexanderi. Kontyû 46:433–438
Aoki S, Akimoto S, Yamane S (1981) Observations on Pseudoregma alexanderi (Homoptera, Pemphigidae), an aphid species producing pseudoscorpion–like soldiers on bamboos. Kontyû 49:355–366
Arakaki N (1992) Shortened longevity of soldiers of the bamboo aphid Pseudoregma koshunensis (Takahashi) (Homoptera: Aphididae) due to attack behavior. J Ethol 10:149–151
Banno H (1997) Population interaction between aphidophagous butterfly, Taraka hamada (Lepidoptera, Lycaenidae) and its larval prey aphid, Ceratovacuna japonica. Trans Lepid Soc Japan 48:115–123
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate—a practical and powerful approach to multiple testing. J Roy Stat Soc B-Methodol 57:289–300
Carlin NF, Gladstein DS, Berry AJ, Pierce NE (1994) Absence of kin discrimination behavior in a soldier–producing aphid, Ceratovacuna japonica (Hemiptera: Pemphigidae; Cerataphidini). J NY Entomol Soc 102:287–298
Endler JA (1986) Natural selection in the wild. Princeton University Press, Princeton, p 17
Hattori M, Itino T (2008) Soldiers’ armature changes seasonally and locally in an eusocial aphid (Homoptera: Aphididae). Sociobiology 52:429–436
Hattori M, Kishida O, Itino T (2013) Buying time for colony mates: the anti–predatory function of soldiers in the eusocial aphid Ceratovacuna japonica (Homoptera, Hormaphidinae). Insect Soc. doi:10.1007/s00040-012-0258-2
Ito Y, Tanaka S, Yukawa J, Tsuji K (1995) Factors affecting the proportion of soldiers in eusocial bamboo aphid, Pseudoregma bambucicola, colonies. Ethol Ecol Evol 7:335–345
Johnson MTJ, Agrawal AA, Maron JL, Salminen JP (2009) Heritability, covariation and natural selection on 24 traits of common evening primrose (Oenothera biennis) from a field experiment. J Evolution Biol 22:1295–1307
Kappes H, Sinsch U (2002) Temperature- and predator-induced phenotypic plasticity in Bosmina cornuta and B. pellucid (Crustacea: Cledocera). Fresh Biol 47:1944–1955
Kishida O, Trussell GC, Nishimura K (2007) Geographic variation in a predator–induced defense and its genetic basis. Ecology 88:1948–1954
Kishida O, Trussell GC, Nishimura K (2009a) Top–down effects on antagonistic inducible defense and offense. Ecology 90:1217–1226
Kishida O, Trussell GC, Nishimura K, Ohgushi T (2009b) Inducible defenses in prey intensify predator cannibalism. Ecology 90:3150–3158
Kishida O, Trussell GC, Mougi A, Nishimura K (2010) Evolutionary ecology of inducible morphological plasticity in predator–prey interaction: toward the practical links with population ecology. Popul Ecol 52:37–46
Kishida O, Trussell GC, Ohno A, Kuwano S, Ikawa T, Nishimura K (2011) Predation risk suppresses the positive feedback between size structure and cannibalism. J Anim Ecol 80:1278–1287
Kozarzhevskaya E (1986) Scale insects (Homoptera, Coccoidea) of ornamental plants in the European part of the USSR and some neighboring countries. Entomol Rev 64:144–158
Kurosu U, Aoki S (1994) Gall formation, outsiders and soldiers of the aphid Ceratovacuna japonica (Homoptera). Jpn J Entmol 62:793–802
Lively CM (1986a) Predator–induced shell dimorphism in the acorn barnacle Chthamalus anisopoma. Evolution 40:232–242
Lively CM (1986b) Competition, comparative life histories, and maintenance of shell dimorphism in a barnacle. Ecology 67:858–864
Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA (2005) Ecological consequences of phenotypic plasticity. Trends Ecol Evol 20:685–692
Moran NA (1992) The evolutionary maintenance of alternative phenotypes. Am Nat 139:971–989
Moriuti S (1982) Xyloryctidae. In: Inoue H et al (eds) Moths of Japan, vol 1. Kodansha, Tokyo, p 258
Morris G, Foster WA (2008) Duelling aphids: electrical penetration graphs reveal the value of fighting for a feeding site. J Exp Biol 211:1490–1494
Mougi A, Kishida O (2009) Reciprocal phenotypic plasticity can lead to stable predator–prey interaction. J Anim Ecol 78:1172–1181
Mougi A, Kishida O, Iwasa Y (2011) Coevolution of phenotypic plasticity: why inducible offense is rarer than inducible defense? Evolution 65:1079–1087
Ohara K (1985) Observations on the prey–predator relationship between Pseudoregma bambucicola (Homoptera, Pemphigidae) and Metasyrphus confrater (Diptera, Syrphidae), with special reference to the behaviour of the aphid soldiers. Esakia 23:107–110
Pettersson LB, Brönmark C (1997) Density–dependent costs of an inducible morphological defense in crucian carp. Ecology 78:1805–1815
Pike N, Foster WA (2008) The ecology of altruism in a clonal insects. In: Korb J, Heinze J (eds) Ecology of social evolution. Springer, Berlin, pp 37–56
Pike N, Braendle C, Foster WA (2004) Seasonal extension of the soldier instar as a route to increased defence investment in the social aphid Pemphigus spyrothecae. Ecol Entomol 29:88–95
Shibao H (1998) Social structure and the defensive role of soldiers in a eusocial bamboo aphid, Pseudoregma bambucicola (Homoptera: Aphididae): a test of the defence–optimization hypothesis. Res Popul Ecol 40:325–333
Shibao H (1999) Reproductive schedule and factors affecting soldier production in the eusocial bamboo aphid Pseudoregma bambucicola (Homoptera, Aphididae). Insect Soc 46:378–386
Shibao H, Kutsukake M, Fukatsu T (2004) Density triggers soldier production in a social aphid. Proc R Soc Lond B Biol Sci 271:S71–S74
Shingleton AW, Foster WA (2000) Ant tending influences soldier production in a social aphid. Proc R Soc Lond B Biol Sci 267:1863–1868
Shingleton AW, Foster WA (2001) Behaviour, morphology and the division of labour in two soldier–producing aphids. Anim Behav 62:671–679
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. W. H. Freeman and Company, New York
Stadler B, Dixon AFG (2005) Ecology and evolution of aphid–ant interactions. Annu Rev Ecol Evol Syst 36:345–372
Stern DL, Foster WA (1996) The evolution of soldiers in aphids. Biol Rev 71:27–79
Takahashi R (1958) On the aphids of Ceratovacuna in Japan. Kontyû 26:187–190
Tanaka S, Ito Y (1994) Reversal of caste production schedule in a eusocial aphid, Pseudoregma koshunensis. Naturwissenschaften 81:411–413
Thompson JN, Cunningham BM (2002) Geographic structure and dynamics of coevolutionary selection. Nature 417:735–738
Toju H, Sota T (2006) Imbalance of predator and prey armament: geographic clines in phenotypic interface and natural selection. Am Nat 167:105–117
Tollrian R, Harvell CD (eds) (1999) The ecology and evolution of inducible defenses. Princeton University Press, Princeton
Trussell GC (2000) Predator–induced plasticity and morphological trade–offs in latitudinally separated populations of Littorina obtusata. Evol Ecol Res 2:803–822
Trussell GC, Smith LD (2000) Induced defenses in response to an invading crab predator: an explanation of historical and geographic phenotypic change. Proc Natl Acad Sci USA 97:2123–2127
Van Buskirk J, Relyea RA (1998) Selection for phenotypic plasticity in Rana sylvatica tadpoles. Biol J Linn Soc 65:301–328
Verhoeven KJF, Simonsen KL, McIntyre LM (2005) Implementing false discovery rate control: increasing your power. Oikos 108:643–647
Weisser WW, Braendle C, Minoretti N (1999) Predator–induced morphological shift in the pea aphid. Proc R Soc Lond B Biol Sci 266:1175–1181
Werner EE, Peacor SD (2003) A review of trait–mediated indirect interactions in ecological communities. Ecology 84:1083–1100
Yurista PM (2000) Cyclomorphosis in Daphnia lumholtzi induced by temperature. Fresh biol 43:207–213
Acknowledgments
The authors thank Ms. Yumi Nakadera for helpful discussions. This work was supported by JSPS to M. H. (no. 216649), to O. K. (no. 2277001100), and to T. I. (no. 22570015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hattori, M., Kishida, O. & Itino, T. Soldiers with large weapons in predator-abundant midsummer: phenotypic plasticity in a eusocial aphid. Evol Ecol 27, 847–862 (2013). https://doi.org/10.1007/s10682-012-9628-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-012-9628-5