Abstract
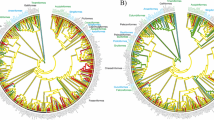
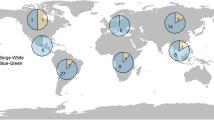
Eggshell structure is related to fundamental aspects of embryonic development (via water and gas exchange), adult ecology and behavior (via nest site selection), and demography (via effects on survival). We compared life-history characteristics between gekkotans that lay rigid- versus parchment- shelled eggs to determine if evolutionary shifts in eggshell structure are associated with life-history evolution. Ancestral gekkotans laid parchment-shelled eggs, with rigid-shelled eggs evolving later. Clutch size in oviparous gekkotans is fixed at one or two eggs, and this characteristic eliminates an egg size versus clutch size tradeoff as a life-history strategy. We found that species laying rigid-shelled eggs exhibit (1) smaller eggs relative to adult body size, (2) smaller hatchlings relative to the size of the egg, (3) earlier embryonic stage at oviposition, (4) longer incubation periods, and (5) smaller adult body sizes than species laying parchment-shelled eggs. These patterns hold when accounting for phylogenetic relatedness, and are not explained by geographic distributions of climate and habitat. In general, our data support the hypothesis that the spherical shapes of rigid-shelled eggs limit their size (volume), which in turn has restricted hatchling size and adult body size. In contrast, while parchment-shelled eggs are similarly constrained in width, elongate shapes allow egg sizes, and hence hatchling sizes, to increase relative to adult body sizes. Finally, the evolution of rigid-shelled eggs may have allowed gekkotans to become so successful; over 1,000 species lay rigid-shelled eggs, as compared to about 200 species that lay eggs exhibiting the ancestral parchment-shelled condition.




Similar content being viewed by others
References
Ackerman RA, Seagrave RC, Dmi’el R, Ar A (1985) Water and heat exchange between parchment-shelled reptile eggs and their surroundings. Copeia 1985:703–711
Andrews RM (1982) Patterns of growth in reptiles. In: Gans C, Pough FH (eds) Biology of the reptilia. Vol 13 Physiology D: physiological ecology. Academic Press, New York, pp 273–320
Andrews R (2004) Patterns of embryonic development. In: Deeming DC (ed) Reptilian incubation: environment, evolution and behaviour. Nottingham University Press, Nottingham, pp 75–102
Annable TJ (2004) Reproductive biology of Nephrurus and Underwoodisaurus gekkotans (Reptilia: Gekkonidae: Carphodactylini). Unpublished PhD thesis, The University of Sydney
Bain MM (1991) A reinterpretation of eggshell strength. In: Solomon SE (ed) Egg and eggshell quality. Wolfe Publishing Limited, Aylesbury, pp 131–145
Bauwens D, Díaz-Uriarte R (1997) Covariation of life-history traits in lacertid lizards: a comparative study. Am Nat 149:91–111
Belinsky A, Ackerman RA, Dmi’el R, Ar A (2004) Water in reptilian eggs and hatchlings. In: Deeming DC (ed) Reptilian incubation: environment, evolution and behaviour. Nottingham University Press, Nottingham, pp 125–141
Bomford M, Kraus F, Barry SC, Lawrence E (2009) Predicting establishment success for alien reptiles and amphibians: a role for climate matching. Biol Inv 11:713–724
Bond JE, Opell BD (1998) Testing adaptive radiation and key innovation hypotheses in spiders. Evolution 52:403–414
Branch B (1998) Field guide to snakes and other reptiles of Southern Africa. Ralph Curtis Publishing, Florida
Brown WC, Alcala AC (1957) Viability of lizard eggs exposed to sea water. Copeia 1957:39–41
Brown SG, Duffy PK (1992) The effects of egg-laying site, temperature, and salt water on incubation time and hatching success in the gekkotan Lepidodactylus lugubris. J Herpetol 26:510–513
Carranza S, Arnold EN, Mateo JA, Lopez-Jurado LF (2000) Long-distance colonization and radiation in gekkonid lizards, Tarentola (Reptilia: Gekkonidae), revealed by mitochondrial DNA sequences. Proc R Soc Lond B 267:637–649
Congdon JD, Gibbons JW (1987) Morphological constraint on egg size: a challenge to optimal egg size theory? Proc Natl Acad Sci 84:4145–4147
Cronin ER, Seymour RS (2000) Respiration of the eggs of the giant cuttlefish Sepia apama. Marine Biol 136:863–870
Deeming DC (1988) Eggshell structure of lizards of two sub-families of the Gekkonidae. Herpetol J 1:230–234
Deeming DC, Thompson MB (1991) Gas exchange across reptilian eggshells. In: Deeming DC, Ferguson MWJ (eds) Egg incubation: its effects on embryonic development in birds and reptiles. Cambridge University Press, Cambridge, pp 277–284
Deeming DC, Unwin DM (2004) Reptilian incubation: evolution and the fossil record. In: Deeming DC (ed) Reptilian incubation: environment, evolution and behaviour. Nottingham University Press, Nottingham, pp 1–14
Doughty P (1997) The effects of “fixed” clutch sizes on lizard life-histories: reproduction in the Australian velvet gekkotan, Oedura lesueurii. J Herpetol 31:266–272
Dufaure JP, Hubert J (1961) Table de de’veloppment du le’zard vivipare: Lacerta (Zootica) vivipara Jaquin. Arch Anat Microsc Mo 50:309–328
Dunson WA (1982) Low water vapor conductance of hard-shelled eggs of the gekkotan lizards Hemidactylus and Lepidodactylus. J Exp Zool 219:377–379
Felsenstein J (1997) Phylogenies and the comparative method. Am Nat 125:1–15
Gamble T, Bauer AM, Greenbaum E, Jackman TR (2008) Out of the blue: a novel trans-Atlantic clade of gekkotans (Gekkota, Squamata). Zool Scrip 37:355–366
Gardner AS (1985) Viability of the eggs of the day-gekkotan Phelsuma sundbergi exposed to sea water. Br J Herpetol 6:435–436
Greer AE (1989) The biology and evolution of Australian lizards. Surrey Beatty, New South Wales
Henkel WF, Schmidt W (1995) Geckoes: biology, husbandry and reproduction. Krieger Publishing, Florida
Henle K (1990) Population ecology and life history of arboreal gecko Gehyra variegata in arid Australia. Herpetol Monogr 4:30–60
Ineich I (2010) How habitat disturbance benefits gekkotans: conservation implications. Comptes Rendus Biol 444:76–82
Iverson JB, Ewert MA (1991) Physical characteristics of reptilan eggs and a comparison with avian eggs. In: Deeming DC, Ferguson MWJ (eds) Egg incubation: its effects on embryonic development in birds and reptiles. Cambridge University Press, Cambridge, pp 87–100
Köhler G (2005) Incubation of reptile eggs. Krieger Publishing, Florida
Kratochvíl L, Frynta D (2006) Egg shape and size allometry in gekkotans (Squamata: Gekkota), lizards with contrasting eggshell structure: why lay spherical eggs? J Zool Syst Evol Res 44:217–222
Kratochvíl L, Kubicka L (2007) Why reduce clutch size to one or two eggs? Reproductive allometries reveal different evolutionary causes of invariant clutch size in lizards. Funct Ecol 21:171–177
Maddison WP, Maddison DR (2006) Mesquite: a modular system for evolutionary analysis. Version 1.1. http://www.mesquiteproject.org
Michoud EJ, Echternacht AC (1995) Geographic variation in the life history of the lizard Anolis carolinensis and support for the pelvic constraint model. J Herpetol 29:86–97
Midford PE, Garland T Jr, Maddison WP (2005) PDAP package of mesquite. Version 1.07
Moreira PL, Barata M (2005) Egg mortality and early embryo hatching caused by fungal infection of Iberian rock lizard (Lacerta monticula) clutches. Herpetol J 15:265–272
Oftedal OT (2002) The origin of lactation as a water source for parchment-shelled eggs. J Mammary Gland Biol Neoplasia 17:253–266
Osadnik G (1984) An investigation of egg laying in Phelsuma (Reptilia: Sauria: Gekkonidae). Ampibia-Reptilia 5:125–134
Packard GC (1991) The physiological and ecological importance of water to embryos of oviparous reptiles. In: Deeming DC, Ferguson MWJ (eds) Egg incubation: its effects on embryonic development in birds and reptiles. Cambridge University Press, Cambridge, pp 213–228
Packard MJ, DeMarco VG (1991) Eggshell structure and formation in eggs of oviparous reptiles. In: Deeming DC, Ferguson MWJ (eds) Egg incubation: its effects on embryonic development in birds and reptiles. Cambridge University Press, Cambridge, pp 53–69
Packard GC, Packard MJ (1988) The physiological ecology of reptilian eggs and embryos. In: Gans C, Huey RB (eds) Biology of the reptilia, vol 16, defense and life history, Alan R. Liss Inc., New York, pp 523–605
Packard MJ, Seymour RS (1997) Evolution of the amniote egg. In: Sumida SS, Martin KLM (eds) Amniote origins: completing the transition to land. Academic Press, New York, pp 265–290
Pike DA, Webb JK, Shine R (2010) Nesting in a thermally challenging environment: nest site selection in a rock-dwelling gecko, Oedurea lesueurii (Reptilia: Gekkonidae). Biol J Linn Soc 99:250–259
Pough FH, Andrews RM, Cadle JE, Crump ML, Savitzky AH, Wells KD (2004) Herpetology. Prentice-Hall, New Jersey
Schleich HH, Kästle W (1988) Reptile egg-shells SEM stlas. Gustav Fischer, Stuttgart
Shattuck M, Williams SA (2010) Arboreality has allowed for the evolution of increased longevity in mammals. P Natl Acad Sci USA 107:4635–4639
Shedlock AM, Edwards SV (2009) Amniotes (Amniota). In: Hedges SB, Kumar S (eds) Timetree of life. Oxford University Press, Oxford, pp 375–379
Simbotwe MP (1983) Comparative ecology of diurnal gekkotans (Lygodactylus) in Kafue flats, Zambia. Afr J Ecol 21:143–153
Sinervo B, Licht P (1991) Proximate constraints on the evolution of egg size, number, and total clutch mass in lizards. Science 252:1300–1302
Stewart JR (1997) Morphology and evolution of the egg of oviparous amniotes. In: Sumida SS, Martin KLM (eds) Amniote origins: completing the transition to land. Academic Press, New York, pp 291–326
Thompson MB, Russell KJ (1999) Growth and energetics of embryos of the gekkotan, Phyllodactylus marmoratus, a species with hard-shelled eggs. Herpetol J 9:37–42
Thompson MB, Speake BK (2004) Egg morphology and composition. In: Deeming DC (ed) Reptilian incubation: environment, evolution and behaviour. Nottingham University Press, Nottingham, pp 45–74
Warne RW, Charnov EL (2008) Reproductive allometry and the size-number trade-off for lizards. Am Nat 172:E80–E98
Wise PAD, Vickaryous MK, Russell AP (2009) An embryonic staging table for in ovo development of Eublepharis macularius, the leopard gecko. Anat Rec 292:1198–1212
Xu D–D, Ji X (2007) Sexual dimorphism, female reproduction and egg incubation in the oriental leaf-toed gekkotan (Hemidactylus bowringii) from southern China. Zoology 110:20–27
Zhang Y-P, Du W-G, Zhu L-J (2009) Differences in body size and female reproductive traits between two sympatric gekkotans: Gekko japonicus and Gekko hokouensis. Folia Zool 58:113–122
Zhao EM, Zhao KT, Zhou KY (1999) Fauna Sinica Reptilia, vol 2: Squamata. Chinese Science Press, Beijing
Acknowledgments
We thank Rick Shine for encouragement and providing crucial references and Aaron Bauer for kindly providing access to unpublished phylogenetic trees. Aaron Bauer and Fred Janzen provided helpful comments that substantially improved the manuscript. We are grateful to L. Kratochvíl, T. Annable, M. Thompson and X. Ji for sharing their datasets on gekkotan life history, and H. Ye for assistance with data collection. This work was supported by grants from the Natural Science Foundation of China (30770274) and the University of Sydney (to W.-G.D.); by NSF award DEB-0844523 and a 2009 Visiting Fellowship for Women from the School of Biological Sciences, University of Sydney (to R.M.A.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pike, D.A., Andrews, R.M. & Du, WG. Eggshell morphology and gekkotan life-history evolution. Evol Ecol 26, 847–861 (2012). https://doi.org/10.1007/s10682-011-9527-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-011-9527-1