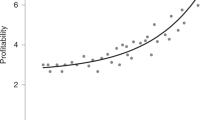
Abstract
Lek systems, where females often use centrality to assess male quality, highlight a general paradox in evolutionary biology: how can female preferences for males providing good genes persist when consequential strong directional selection is predicted to deplete additive genetic variance in male quality and thereby obliterate benefits of choosiness? An explanation contributing to the resolution of this lek paradox may be that genetic variance is retained when an indirect mate choice cue, such as centrality on a lek, is an imperfect indicator of male genetic quality. Here I investigate whether the presence of alternative male mating tactics limits the reliability of centrality as a quality indicator in lek-breeding topi antelopes. Whereas males establishing territories directly on the central lek were relatively large, smaller peripheral males regularly shifted their territories centripetally and in this way also occasionally obtained central territories. By such opportunistic queuing, small males could increase their mating success drastically; however, their territorial tenure in the lek centre was relatively short, consistent with moderate competitive ability. These results suggest that male topi antelopes can obtain central lek territories through alternative mating tactics, providing scope for variance in male quality on the central lek. In a separate finding, the mating success of central males was found to increase during territorial tenure, independent of estimated age. The demonstration of queuing in both space and time on a mammalian lek highlights the importance of considering male tactical dynamics over time in order to avoid an inflated appearance of the lek paradox.


Similar content being viewed by others
References
Ahnesjö I, Kvarnemo C, Merilaita S (2001) Using potential reproductive rates to predict mating competition among individuals qualified to mate. Behav Ecol 12:397–401
Anthony AJ, Lightfoot CJ (1984) Field determination of age and sex in tsessebe Damaliscus lunatus. S Afr J Wildl Res 14:19–22
Apollonio M, Festa-Bianchet M, Mari F, Riva M (1990) Site-specific asymmetries in male copulatory success in a fallow deer lek. Anim Behav 39:205–212
Apollonio M, Festa-Bianchet M, Mari F, Mattioli S, Sarno B (1992) To lek or not to lek—mating strategies of male fallow deer. Behav Ecol 3:25–31
Bakker TCM, Pomiankowski A (1995) The genetic basis of female mate preferences. J Evol Biol 8:129–171
Balmford A (1990) Lekking in Uganda kob. PhD thesis, University of Cambridge
Balmford A, Albon S, Blakeman S (1992) Correlates of male mating success and female choice in a lek-breeding antelope. Behav Ecol 3:112–123
Beehler BM, Foster MS (1988) Hotshots, hotspots, and female preference in the organization of lek mating systems. Am Nat 131:203–219
Bercovitch FB, Loomis CP, Rieches RG (2009) Age-specific changes in reproductive effort and terminal investment in female Nile lechwe. J Mammal 90:40–46
Borgia G (1979) Sexual selection and the evolution of mating systems. In: Blum MS, Blum NA (eds) Sexual selection and reproductive competition. Academic Press, New York, pp 19–80
Bradbury JW (1981) The evolution of leks. In: Alexander RD, Tinkle DW (eds) Natural selection and social behaviour. Chiron Press, New York, pp 138–169
Bradbury JW, Vehrencamp SL (1998) Principles of animal communication. Sinauer, Sunderland
Bro-Jørgensen J (2001) Lek-breeding in topi antelopes (Damaliscus lunatus). PhD thesis, University of London
Bro-Jørgensen J (2002) Overt female mate competition and preference for central males in a lekking antelope. Proc Natl Acad Sci USA 99:9290–9293
Bro-Jørgensen J (2003) No peace for estrous topi cows on leks. Behav Ecol 14:521–525
Bro-Jørgensen J (2007a) The intensity of sexual selection predicts weapon size in male bovids. Evolution 61:1316–1326
Bro-Jørgensen J (2007b) Reversed sexual conflict in a promiscuous antelope. Curr Biol 17:2157–2161
Bro-Jørgensen J (2008) The impact of lekking on the spatial variation in payoffs to resource-defending topi bulls (Damaliscus lunatus). Anim Behav 75:1229–1234
Bro-Jørgensen J (2011) Intra- and intersexual conflicts and cooperation in the evolution of mating strategies: lessons learnt from ungulates. Evol Biol 38:28–41
Bro-Jørgensen J, Durant SM (2003) Mating strategies of topi bulls: getting in the centre of attention. Anim Behav 65:585–594
Byers JA, Waits L (2006) Good genes sexual selection in nature. Proc Natl Acad Sci USA 103:16343–16345
Clutton-Brock TH, Guinness FE, Albon SD (1982) Red deer—behaviour and ecology of two sexes. Edinburgh University Press, Edinburgh
Clutton-Brock TH, Hiraiwa-Hasegawa M, Robertson A (1989) Mate choice on fallow deer leks. Nature 340:463–465
Cornwallis CK, Uller T (2010) Towards an evolutionary ecology of sexual traits. Trends Ecol Evol 25:145–152
East ML, Hofer H (2001) Male spotted hyenas (Crocuta crocuta) queue for status in social groups dominated by females. Behav Ecol 12:558–568
East ML, Burke T, Wilhelm K, Greig C, Hofer H (2003) Sexual conflicts in spotted hyenas: male and female mating tactics and their reproductive outcome with respect to age, social status and tenure. Proc R Soc Lond B 270:1247–1254
Falconer DS (1981) Introduction to quantitative genetics, 2nd edn. Longman, London
Gosling LM, Petrie M (1990) Lekking in topi: a consequence of satellite behavior by small males at hotspots. Anim Behav 40:272–287
Hall MD, Lailvaux SP, Blows MW, Brooks RC (2010) Sexual conflict and the maintenance of multivariate genetic variation. Evolution 64:1697–1703
Höglund J, Alatalo RV (1995) Leks. Princeton University Press, Princeton, New Jersey
Höglund J, Robertson JGM (1990) Female preferences, male decision rules and the evolution of leks in the great snipe (Gallinago media). Anim Behav 40:15–22
Hunt J, Bussiere LF, Jennions MD, Brooks R (2004) What is genetic quality? Trends Ecol Evol 19:329–333
Isvaran K, Jhala Y (2000) Variation in lekking costs in blackbuck (Antilope cervicapra): relationship to lek-territory location and female mating patterns. Behaviour 137:547–563
Jewell PA (1972) Social organisation and movements of topi (Damaliscus korrigum) during the rut, at Ishasha, Queen Elizabeth Park, Uganda. Zool Afr 7:233–255
Johnson T, Barton N (2005) Theoretical models of selection and mutation on quantitative traits. Philos Trans R Soc B 360:1411–1425
Kokko H, Johnstone RA (1999) Social queuing in animal societies: a dynamic model of reproductive skew. Proc R Soc Lond B 266:571–578
Kokko H, Lindström J, Alatalo RV, Rintamäki PT (1998) Queuing for territory positions in the lekking black grouse (Tetrao tetrix). Behav Ecol 9:376–383
Kotiaho JS, Simmons LW, Tomkins JL (2001) Towards a resolution of the lek paradox. Nature 410:684–686
Kotiaho JS, Lebas NR, Puurtinen M, Tomkins JL (2008) On the resolution of the lek paradox. Trends Ecol Evol 23:1–3
McElligott AG, Altwegg R, Hayden TJ (2002) Age-specific survival and reproductive probabilities: evidence for senescence in male fallow deer (Dama dama). Proc R Soc Lond B 269:1129–1137
Nefdt RJC (1992) Lek-breeding in Kafue lechwe. PhD thesis, University of Cambridge
Nefdt RJC, Thirgood SJ (1997) Lekking, resource defense, and harassment in two subspecies of lechwe antelope. Behav Ecol 8:1–9
Nussey DH, Kruuk LEB, Morris A, Clements MN, Pemberton JM, Clutton-Brock TH (2009) Inter- and intrasexual variation in aging patterns across reproductive traits in a wild red deer population. Am Nat 174:342–357
Pelletier F, Festa-Bianchet M (2006) Sexual selection and social rank in bighorn rams. Anim Behav 71:649–655
Petrie M, Roberts G (2007) Sexual selection and the evolution of evolvability. Heredity 98:198–205
Pomiankowski A, Møller AP (1995) A resolution of the lek paradox. Proc R Soc Lond B 260:21–29
Radwan J (2008) Maintenance of genetic variation in sexual ornaments: a review of the mechanisms. Genetica 134:113–127
Saether SA, Baglo R, Fiske P, Ekblom R, Höglund J, Kålås A (2005) Direct and indirect mate choice on leks. Am Nat 166:145–157
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Tomkins JL, Radwan J, Kotiaho JS, Tregenza T (2004) Genic capture and resolving the lek paradox. Trends Ecol Evol 19:323–328
Wade MJ (1979) Sexual selection and variance in reproductive success. Am Nat 114:742–764
Weckerly FW (1998) Sexual-size dimorphism: influence of mass and mating systems in the most dimorphic mammals. J Mammal 79:33–52
Wiley RH, Poston J (1996) Perspective: indirect mate choice, competition for mates, and coevolution of the sexes. Evolution 50:1371–1381
Wilson AJ, Nussey DH (2010) What is individual quality? An evolutionary perspective. Trends Ecol Evol 25:207–214
Wilson AJ, Kruuk LEB, Coltman DW (2005) Ontogenetic patterns in heritable variation for body size: using random regression models in a wild ungulate population. Am Nat 166:E177–E192
Acknowledgments
I thank the Ministry of Science and Technology in Kenya, Narok County Council, the Senior Warden of Masai Mara National Reserve, the Management of the Olare Orok Conservancy and Kenya Wildlife Service for permission to do field work, Mada Hotels (T. Mhajan) for logistic support, and Simone Ciuti and two anonymous reviewers for valuable comments. This research was supported by Marie Curie, ZSL and RCUK fellowships to the author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bro-Jørgensen, J. Queuing in space and time reduces the lek paradox on an antelope lek. Evol Ecol 25, 1385–1395 (2011). https://doi.org/10.1007/s10682-011-9523-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-011-9523-5