Abstract
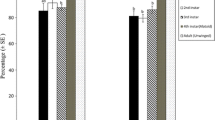
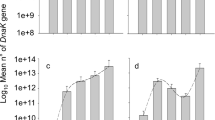
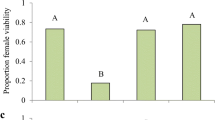
Species belonging to the same guild (i.e. sharing the same resources) can reduce the negative effects of resource competition through niche partitioning. Coexisting species may differ in their resource exploitation and in the associated allocation of nutrients, depending on their resource niche. Trade-offs in nutrient allocation, such as between reproduction and survival, or between early and late reproduction, are moderated by the abundance and distribution of resources. In this study we investigate differences in larval resource exploitation and adult reproductive strategy of two sympatric aphid parasitoids sharing a common host. The habitat specialist Aphidius rhopalosiphi and the generalist Aphidius avenae occur in cereal crops of Western Europe, where both species attack the major host resource: the grain aphid Sitobion avenae. For this purpose, we measured their acquisition of capital lipid resources, their age-specific fecundity and reproductive effort, their life span and their metabolic rate. We found that these species do not differ neither in larval lipid accumulation nor in the number of eggs at emergence and the timing of egg production, but diverge in other adult reproductive strategies. The rate of adult egg production was higher in A. rhopalosiphi than A. avenae, but at the expense of producing smaller eggs. Throughout adult life, reproductive effort was higher in A. avenae, perhaps facilitated by its higher metabolic rate than A. rhopalosiphi. The divergence between species in life history syndromes likely reflects their adaptations to their resource niche. A high egg production probably allows the specialist A. rhopalosiphi to exploit more S. avenae individuals in cereal crops, while the generalist A. avenae because of its variety of hosts, maximizes the investment per egg but at the expense of a lower lifespan. Our results suggest that differential resource allocation may be a more common pattern that promotes coexistence of species within a guild.






Similar content being viewed by others
References
Askew RR, Shaw MR (1986) Parasitoid communities: their size structure and development. In: Waage J, Greathead D (eds) Insect parasitoids. London Academic Press, London, pp 225–264
Begon M, Parker GA (1986) Should egg size and clutch size decrease with age? Oïkos 47:293–302
Bernardo J (1996) The particular maternal effect of propagule size, especially egg size: patterns, models, quality of evidence and interpretations. Am Zool 36:216–236
Bezemer TM, Harvey JA, Mills NJ (2005) Influence of adult nutrition on the relationship between body size and reproductive parameters in a parasitoid wasp. Ecol Entomol 30:571–580
Boggs CL (1997) Dynamics of reproductive allocation from juvenile and adult feeding: radiotracer studies. Ecology 78:192–202
Boivin G, Gauvin MJ (2009) Egg size affects larval performance in a coleopteran parasitoid. Ecol Entomol 34:240–245
Bonsall MB, Jansen VAA, Hassel MP (2004) Life history trade–offs assemble ecological guilds. Science 306:111–113
Carton Y, Boulétreau M, van A1phen JJM, van Lenteren JC (1986) The Drosophila parasitic wasps. In: The genetics and biology of Drosophila, vol 3. Academic Press Inc, London, pp 347–394
Chapman RF (1998) The insects: structure and function, 4th edn. Cambridge University Press, Cambridge
Chase JM, Leibold MA (2003) Ecological niches linking classical and contemporary approaches. University of Chicago Press, Chicago
Chesson P (2000) Mechanisms of maintenance of species diversity. Ann Rev Ecol Syst 31:343–366
Clutton-Brock TH, Godfray C (1991) Parental investment. In: Krebs JR, Davies NB (eds) Behavioural ecology: an evolutionary approach. Blackwell Scientific, Oxford, pp 234–262
Colinet H, Salin C, Boivin G, Hance TH (2005) Host age and fitness–related traits in a koinobiont aphid parasitoid. Ecol Entomol 30:473–479
Eijs IEM, van Alphen JJM (1999) Life history correlations: why are hymenopteran parasitoids an exception? Ecol Lett 2:27–35
Ellers J (1996) Fat and eggs: an alternative method to measure the trade–off between survival and reproduction in insect parasitoids. Neth J Zool 46:227–235
Ellers J, Jervis MA (2003) Body size and the timing of egg production in parasitoid wasps. Oïkos 102:164–172
Ellers J, Jervis MA (2004) Why are so few parasitoid wasp species pro–ovigenic? Evol Ecol Res 6:993–1002
Ellers J, van Alphen JJM (1997) Life history evolution in Asobara tabida: plasticity in allocation of fat reserves to survival and reproduction. J Evol Biol 10:771–785
Ellers J, Sevenster JG, Driessen G (2000) Egg load evolution in parasitoids. Am Nat 156:650–665
Fox CW, Czesak ME (2000) Evolutionary ecology of progeny size in arthropods. Ann Rev Entomol 45:41–369
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251
Giron D, Casas J (2003) Mothers reduce egg provisioning with age. Ecol Lett 6:273–277
Godfray HJC (1994) Parasitoids behavioral and evolutionary ecology. Princeton University Press, Princeton
Harvey JA (2008) Comparing and contrasting development and reproductive strategies in the pupal hyperparasitoids Lysibia nana and Gelis agilis (Hymenoptera: Ichneumonidae). Evol Ecol 22:153–166
Harvey JA, Strand MR (2002) The developmental strategies of endoparasitoid wasps vary with host feeding ecology. Ecology 83:2349–2451
Harvey JA, Witjes LMA (2005) Comparing and contrasting life history and development strategies in the pupal hyperparasitoids Lysibia nana and Gelis agilis (Hymenoptera: Ichneumonidae). Appl Entomol Zool 40:309–316
Harvey JA, Bezemer TM, Gols R, Nakamatsu Y, Tanaka T (2008) Comparing the physiological effects and function of larval feeding in closely–related endoparasitoids (Braconidae: Microgastrinae). Physiol Entomol 33:217–225
Harvey JA, Wagenaar R, Bezemer TM (2009) Life-history traits in closely related secondary parasitoids sharing the same primary parasitoid host: evolutionary opportunities and constraints. Entomol Exp Appl 132:155–164
Jervis MA, Ferns PN (2004) The timing of egg maturation in insects: ovigeny index and initial egg load as measures of fitness and of resource allocation. Oïkos 107:449–460
Jervis MA, Heimpel GE, Ferns PN, Harvey JA, Kidd NAC (2001) Life–history strategies in parasitoid wasps: a comparative analysis of ‘ovigeny’. J Anim Ecol 70:442–458
Jervis MA, Ferns PN, Heimpel GE (2003) Body size and the timing of egg production in parasitoid wasps: a comparative analysis. Func Ecol 17:375–383
Khambhampati S, Volkl W, Mackauer M (2000) Phylogenetic relationships among genera of Aphidiinae (Hymenoptera: Braconidae) based on DNA sequence of the mitochondrial 16S rRNA gene. Syst Entomol 25:437–445
Kraaijeveld AR, van Alphen JJM (1994) Geographical variation in resistance of the parasitoid Asobara tabida against encapsulation by Drosophila melanogaster larvae: the mechanism explored. Physiol Entomol 19:9–14
Krespi L (1990) Etude de la biocénose parasitaire des pucerons des céréales dans le bassin de Rennes: cas particulier d’Aphidius uzbekistanicus Luz. PhD thesis, University of Rennes, Rennes, France
Lalonde RG (2005) Egg size variation does not affect offspring performance under intraspecific competition in Nasonia vitripennis, a gregarious parasitoid. J Anim Ecol 74:630–635
Le Lann C, Roux O, Serain N, van Alphen JJM, Vernon P, van Baaren J (2011a) Thermal tolerance of sympatric hymenopteran parasitoid species: does it match their seasonal activities? Physiol Entomol 36:21–28
Le Lann C, Wardziak T, van Baaren J, van Alphen JJM (2011b) Thermal plasticity of metabolic rates linked to life history traits and foraging behaviour in a parasitic wasp. Func Ecol 25:641–651
MacArthur RH, Levins R (1967) The limiting similarity convergence and divergence of coexisting species. Am Nat 101:377–385
Outreman Y, Le Ralec A, Plantegenest M, Chaubet B, Pierre JS (2001a) Superparasitism limitation in an aphid parasitoid: cornicle secretion avoidance and host discrimination ability. J Insect Physiol 47:339–348
Outreman Y, Le Ralec A, Wajnberg E, Pierre JS (2001b) Can imperfect host discrimination explain partial patch exploitation in parasitoids? Ecol Entomol 26:271–280
Pelosse P, Bernstein C, Desouhant E (2007) Differential energy allocation as an adaptation to different habitats in the parasitic wasp Venturia canescens. Evol Ecol 21:669–685
Pelosse P, Amat I, Bernstein C, Desouhant E (2010) The dynamics of energy allocation in adult arrhenotokous and thelytokous Venturia canescens. Entomol Exp Appl 135:68–76
Pexton JJ, Mayhew PJ (2002) Siblicide and life–history evolution in parasitoids. Behav Ecol 13:690–695
Price PW (1972) Parasitoids utilizing the same host: adaptive nature of differences in size and form. Ecology 53:190–195
R Development Core Team (2010) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. ISBN 3–900051–07–0 [WWW document] Available from URL http://www.R-project.org. Accessed Nov 2010
Richard R, Casas J (2009) Complementary roles of nutrient sources of varying stochasticity and controllability in foraging: host-feeding and egg resorption in parasitoids. Ecol Monograph 79:465–483
Rivero A, Casas J (1999) Rate of nutrient allocation to egg production in a parasitic wasp. Proc Roy Soc B-Biol Sci 266:1169–1174
Rivero A, West SA (2002) The physiological costs of being small in a parasitic wasp. Evol Ecol Res 4:407–420
Rivero A, Giron D, Casas J (2001) Lifetime allocation of juvenile and adult nutritional resources to egg production in a holometabolous insect. Proc Roy Soc B-Biol Sci 268:1231–1237
Roberts HLS, Schmidt O (2004) Lifetime egg maturation by host–deprived Venturia canescens. J Insect Physiol 50:195–202
Roff DA (2002) Life history evolution. Sinauer Associates Sunderland
Rosenheim JA (1999) Characterizing the cost of oviposition in insects: a dynamic model. Evol Ecol 13:141–165
Rosenheim JA, Heimpel GE, Mangel M (2000) Egg maturation, egg resorption and the costliness of transient egg limitation in insects. Proc Roy Soc B-Biol Sci 267:1565–1573
Starý P (1970) Biology of aphid parasites (Hymenoptera: Aphidiidae) with respect to integrated control. The Hague, Göttingen
Starý P (1974) Taxonomy, origin, distribution and host range of Aphidius species (Hym., Aphidiidae) in relation to biological control of the pea aphid in Europe and North America. Z Ang Ent 77:141–171
Stavraki-Paulopoulou HG (1966) Contribution à l’étude de la capacité reproductrice et de la fécondité réelle d’Opius concolor. Ann Epiphy 17:391–435
Stilmant D, Van Bellinghen C, Hance T, Boivin G (2008) Host specialization in habitat specialists and generalists. Oecologia 156:905–912
Tilman D (1982) Ressource competition and community structure. Princeton University Press, Princeton
van Baaren J, Héterier V, Hance T, Krespi L, Cortesero AM, Poinsot D, Le Ralec A, Outreman Y (2004) Playing the hare or the tortoise in parasitoids: could different oviposition strategies have an influence in host partitioning in two Aphidius species? Ethol Ecol Evol 16:231–242
van Baaren J, Le Lann C, Pichenot J, Pierre JS, Krespi L, Outreman Y (2009) How could host discrimination abilities influence the structure of a parasitoid community? Bull Ent Res 99:299–306
van Noordwijk AJ, de Jong G (1986) Acquisition and allocation of resources: their influence on variation in life history tactics. Am Nat 128:137–142
Vinson SB, Iwantsch GF (1980) Host regulation by insect parasitoids. Q Rev Biol 55:143–165
Visser B, Ellers J (2008) Lack of lipogenesis in parasitoids: a review of physiological mechanisms and evolutionary implications. J Insect Physiol 54:1315–1322
Visser B, Le Lann C, den Blanken FJ, Harvey JA, van Alphen JJM, Ellers J (2010) Loss of lipid synthesis as an evolutionary consequence of a parasitic lifestyle. Proc Nat Acad Sci USA 107:8677–8682
Acknowledgments
All of the experiments conducted in this study comply with French and Dutch legal code requirements. We thank Nicolas Chazot for technical assistance with egg size measurements. We are grateful to the people of UMR INRA ESE ‘Ecologie et Santé des Ecosystèmes’, for hosting us for some experiments and especially to Marc Roucaute and Dominique Huteau for providing access and help with the ultra-precision balance. This research was supported by a grant to C.L.L. from the Ministère de l’Enseignement Supérieur et de la Recherche and a travel and research grant from Rennes Métropole, by the COMPAREVOL program (Marie Curie Excellence Chair, http://comparevol.univ-rennes1.fr/) and by the ECOCLIM program founded by the Région Bretagne. B.V. was funded by Netherlands Organisation for Scientific Research (NWO) ALW Grant 816.01.013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Le Lann, C., Visser, B., van Baaren, J. et al. Comparing resource exploitation and allocation of two closely related aphid parasitoids sharing the same host. Evol Ecol 26, 79–94 (2012). https://doi.org/10.1007/s10682-011-9498-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-011-9498-2